Why Your Generic Drug Still Isn’t on the Shelf
You see the news: the FDA approved a cheaper version of your expensive prescription. You’re relieved. But when you walk into the pharmacy, it’s still not there. That’s not a glitch. It’s the system. Between 2023 and 2025, generic drug delays became more common-not because of manufacturing problems or shortages, but because of legal maneuvers that have nothing to do with safety or science.
The 30-Month Lockout
When a generic company wants to launch a cheaper version of a brand-name drug, it files what’s called a Paragraph IV certification. That’s a legal notice saying: ‘This patent is invalid, or we don’t infringe it.’ The brand-name company then has 45 days to sue. If they do, the FDA is legally forced to delay final approval for 30 months. That’s not a review period. It’s a court-imposed hold.
That 30-month clock doesn’t reset if the patent is weak. It doesn’t pause if the case is clearly frivolous. It just runs. And in 2024, 68% of all generic applications included this kind of challenge. That means nearly seven in ten generic drugs hit this legal wall before they even reach the pharmacy.
Patent Thickets Are the Real Problem
Brand-name drug companies don’t rely on one patent anymore. They pile them on. In 2020, the average drug had 12.3 patents listed in the FDA’s Orange Book. By 2025, that number jumped to 14.7. Some drugs now have over 30 patents covering everything from pill coatings to dosing schedules.
This is called patent thicketing. It’s not innovation. It’s obstruction. Take Humira. Its core patent expired in 2016. But through a series of 242 additional patents, the manufacturer blocked generics until 2023. That’s seven extra years of monopoly pricing-$13 billion a year in sales-just by filing lawsuits, not new drugs.
Harvard’s Dr. Aaron Kesselheim put it bluntly: ‘The current system extends monopolies beyond the 20-year patent term by an average of 3.7 years per drug.’
Who Gets Hurt the Most?
It’s not just patients. It’s small generic manufacturers. In 2025, 63% of delayed generic drugs came from companies with annual revenue under $500 million. These firms don’t have the legal teams or war chests to fight multi-year court battles. Big companies like Teva or Sandoz can absorb the cost. Smaller ones often just give up.
And the cost? The average patent lawsuit now runs $12.7 million per case. That’s up from $9.3 million in 2023. For a small company, that’s more than their entire annual budget.
Meanwhile, patients pay the price. A 2025 survey found that 82% of pharmacists get daily calls from patients asking why their approved generic isn’t available. The most common drugs? Eliquis, Trulicity, Steglatro-all drugs where generics were approved but blocked by litigation.

Complex Drugs Get Hit Hardest
Not all generics are created equal. Simple pills? They’re easier to copy. But injectables, inhalers, and complex formulations? They’re harder to make, and the patent system makes them even harder to launch.
In 2025, 89% of delayed complex generics faced patent-related blocks. Compare that to 63% for simple oral tablets. Oncology drugs are the worst. The average delay between FDA approval and market launch for cancer generics? 4.1 years. For heart meds? Just 2.8 years.
And it’s not just patents. Supply chain issues played a role too. Nearly 37% of delayed generics between 2023 and 2025 cited problems getting the active ingredient (API) from overseas factories. But even those delays were often worsened by patent lawsuits-because when a company is already tied up in court, they don’t have the resources to fix their supply chain.
The FDA Can’t Fix This
The FDA approved 63 first generics in late 2025. That’s good news. But the agency has no power to override patent law. Even with new AI tools that cut review times by 22% for non-litigated cases, the 30-month stay still applies. The FDA can speed up paperwork, but it can’t stop a lawsuit.
Dr. Patrizia Cavazzoni, head of the FDA’s drug center, admitted in May 2025: ‘Patent litigation remains outside our regulatory authority.’ She’s right. The FDA can’t force a patent holder to stop suing. All they can do is list patents in the Orange Book-and even that’s messy. Some companies list patents that have nothing to do with the drug’s chemistry, just to trigger the 30-month delay.
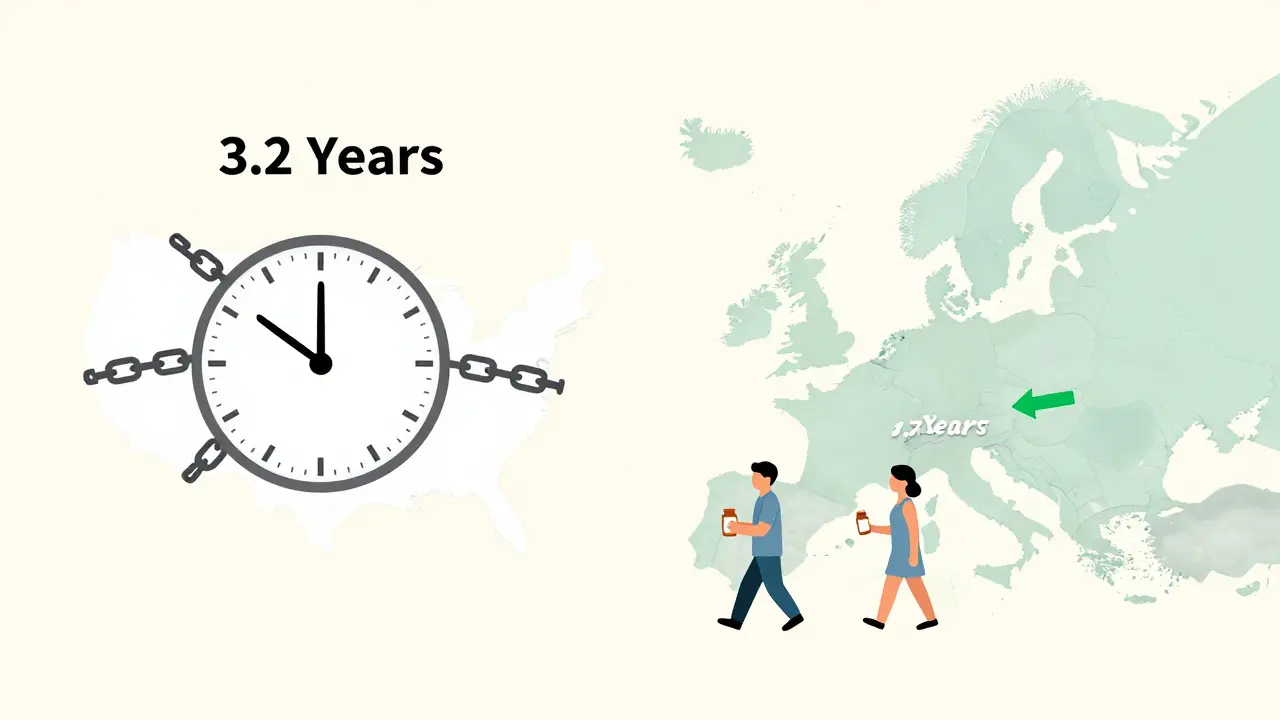
Why Europe Moves Faster
Look across the Atlantic. In Europe, generic drugs hit the market in about 1.7 years after approval. In the U.S.? 3.2 years. Why the difference?
Europe doesn’t have the same 30-month automatic stay. They don’t let patent lawsuits freeze the entire approval process. If a generic is safe and effective, it can launch while litigation plays out. If the brand wins later, they get damages. But patients get access now.
The U.S. system was designed to balance innovation and access. Today, it’s tilted hard toward the former.
What’s Being Done?
There’s pressure to change things. The FTC filed seven enforcement actions between 2024 and 2025 against companies using patent tactics to delay generics. One case against Jazz Pharmaceuticals over Xyrem led to a settlement requiring earlier generic entry.
Congress considered the CREATES Act in 2025. It would make it easier for generic makers to get samples of brand-name drugs to test against-something some companies have refused to provide. But the bill stalled.
Some reformers want to cap the number of patents a company can list per drug. Right now, there’s no limit. In 2025, 41% of generic manufacturers filed petitions with the FDA to remove improper patents from the Orange Book. But those petitions take an average of 18.2 months to resolve. By then, the 30-month clock has already run its course.
The Financial Toll
The cost isn’t just emotional. It’s financial. The Congressional Budget Office estimated that patent delays cost Medicare Part D $3.2 billion in 2025 alone. That’s money spent on brand-name drugs that should have been generics.
Top drugs losing exclusivity in 2025 had combined annual sales of $78.3 billion. That’s a massive opportunity for generics. But because of litigation, only a fraction of that savings reached patients.
Patients aren’t just waiting. They’re skipping doses. Patients For Affordable Drugs Now documented 412 cases between 2023 and 2025 where people couldn’t afford the brand version. One patient paid $487 a month for a drug that should have cost $85. That’s $5,844 a year just to stay alive.
What Comes Next?
There’s no easy fix. The Hatch-Waxman Act, passed in 1984, was meant to encourage generics. Today, it’s being used to block them. The system needs recalibration-not elimination.
Some experts suggest ending the automatic 30-month stay. Let patent cases run in parallel with approval. Let generics enter the market and pay damages if they lose. That’s how most other countries do it.
Others want to limit patent listings to only those that directly protect the drug’s active ingredient. No more coating patents. No more method-of-use patents. Just the real ones.
Until then, patients will keep asking pharmacists: ‘Why isn’t it here yet?’ And the answer won’t be about science. It’ll be about lawsuits.
Why are generic drugs approved by the FDA but still not available?
Even after FDA approval, generic drugs can be blocked by patent lawsuits from brand-name manufacturers. When a generic company challenges a patent, the brand can sue, triggering an automatic 30-month legal hold that prevents the FDA from giving final approval. This delay has nothing to do with safety or quality-it’s purely legal. Between 2023 and 2025, 72% of all generic launch delays were caused by patent litigation, not manufacturing issues or regulatory reviews.
What is a Paragraph IV certification?
A Paragraph IV certification is a legal statement filed by a generic drug maker with the FDA, claiming that a brand-name drug’s patent is invalid or that the generic won’t infringe on it. This triggers the brand company’s right to sue. If they do, the FDA must delay final approval for up to 30 months, regardless of whether the patent is strong or weak. In 2024, 68% of all generic applications included this certification, making it the most common path-and the biggest roadblock-to market entry.
Why do some generic drugs take longer to launch than others?
Complex drugs like injectables, inhalers, and oncology treatments face longer delays because they’re harder to copy and attract more patent challenges. In 2025, 89% of delayed complex generics faced patent-related blocks, compared to 63% for simple pills. Oncology drugs had the longest average delay-4.1 years between FDA approval and market launch. Simple cardiovascular generics took just 2.8 years. The more complex the drug, the more patents brand companies file to block competition.
How do patent thickets delay generic drugs?
Patent thickets are when a single drug has dozens of patents covering minor variations-like pill coatings, dosing schedules, or delivery methods-not the actual active ingredient. Brand companies file these to extend their monopoly. For example, Humira had 242 patents listed after its core patent expired in 2016, blocking generics until 2023. Each patent can trigger a new lawsuit, resetting or extending the 30-month delay. This tactic has extended monopolies by an average of 3.7 years per drug, according to Harvard researchers.
Can the FDA do anything to speed up generic approvals when patents are involved?
No. The FDA can approve a generic drug based on safety and effectiveness, but it cannot override patent law. Even with faster review times thanks to AI tools, the agency is legally required to wait out the 30-month stay triggered by a patent lawsuit. The FDA can remove improper patents from its Orange Book list, but the process takes over a year and doesn’t stop lawsuits already filed. Patent litigation is handled by courts, not regulators.
Why are small generic companies hit harder by patent delays?
Small generic manufacturers often lack the legal resources to fight multi-year patent battles. In 2025, 63% of delayed generics came from companies with annual revenue under $500 million. The average patent lawsuit now costs $12.7 million-far beyond what most small firms can afford. Big companies like Teva or Sandoz can absorb these costs. Smaller ones either drop out or go bankrupt. This reduces competition, keeping prices high and limiting patient access.
How much more do patients pay because of these delays?
Patients pay thousands more per year. For example, a drug like Eliquis might cost $500 a month as a brand, but its generic should cost under $100. Because of patent delays, many patients pay the full brand price for years after approval. A 2025 survey found that patients in these situations spent an average of $487 per month on brand drugs instead of $85 for generics. That’s over $5,000 extra per year. Medicare Part D alone spent $3.2 billion more in 2025 because generics weren’t available.
What’s the difference between U.S. and European generic approval timelines?
In the U.S., the average time between FDA approval and market launch is 3.2 years, mostly due to patent litigation. In Europe, it’s just 1.7 years. Why? Europe doesn’t have an automatic 30-month stay. Generics can launch while lawsuits proceed. If the brand wins, they get compensation-but patients get access immediately. The U.S. system prioritizes patent protection over access. Europe balances both.
Are biosimilars affected the same way as generics?
Even more so. Biosimilars-generic versions of biologic drugs-face even more complex patent landscapes. The average number of patents challenged per biosimilar application jumped from 5.2 in 2020 to 9.7 in 2025. The Humira case, with over 200 patents, created a 10-year exclusivity period. While biosimilar approvals are increasing (17 approved by Q3 2025), patent litigation still delays market entry by years, often more than traditional generics.
What reforms are being considered to fix these delays?
Several reforms are under discussion. The CREATES Act would require brand companies to provide drug samples to generic makers, which some have refused to do. Others want to cap the number of patents that can be listed per drug. The FTC has filed enforcement actions against companies using patents to block competition. Some experts suggest eliminating the automatic 30-month stay and letting generics enter the market while lawsuits proceed. But big pharmaceutical groups like PhRMA oppose these changes, arguing they hurt innovation-even though most of these patents aren’t for new drugs, just extensions of old ones.






calanha nevin
January 30, 2026 AT 16:34And the FDA? They’re handcuffed. No amount of AI can fix a law designed to fail patients.
Niamh Trihy
January 31, 2026 AT 21:51Marc Bains
January 31, 2026 AT 23:15Eliana Botelho
February 1, 2026 AT 09:47owori patrick
February 2, 2026 AT 07:02Kimberly Reker
February 2, 2026 AT 22:04Sazzy De
February 4, 2026 AT 00:19Lisa McCluskey
February 5, 2026 AT 16:36Russ Kelemen
February 5, 2026 AT 18:51Sarah Blevins
February 7, 2026 AT 12:58