Every year, thousands of patients are harmed because two drugs sound or look too similar on a prescription label. It’s not a rare mistake. In fact, look-alike drug names cause about 25% of all medication errors in hospitals and pharmacies. You might think, "How hard can it be to tell apart two drug names?" But when you’re rushing between patients, juggling multiple screens, or squinting at a faded printout, even small similarities can be deadly.
What Makes Drug Names Look or Sound the Same?
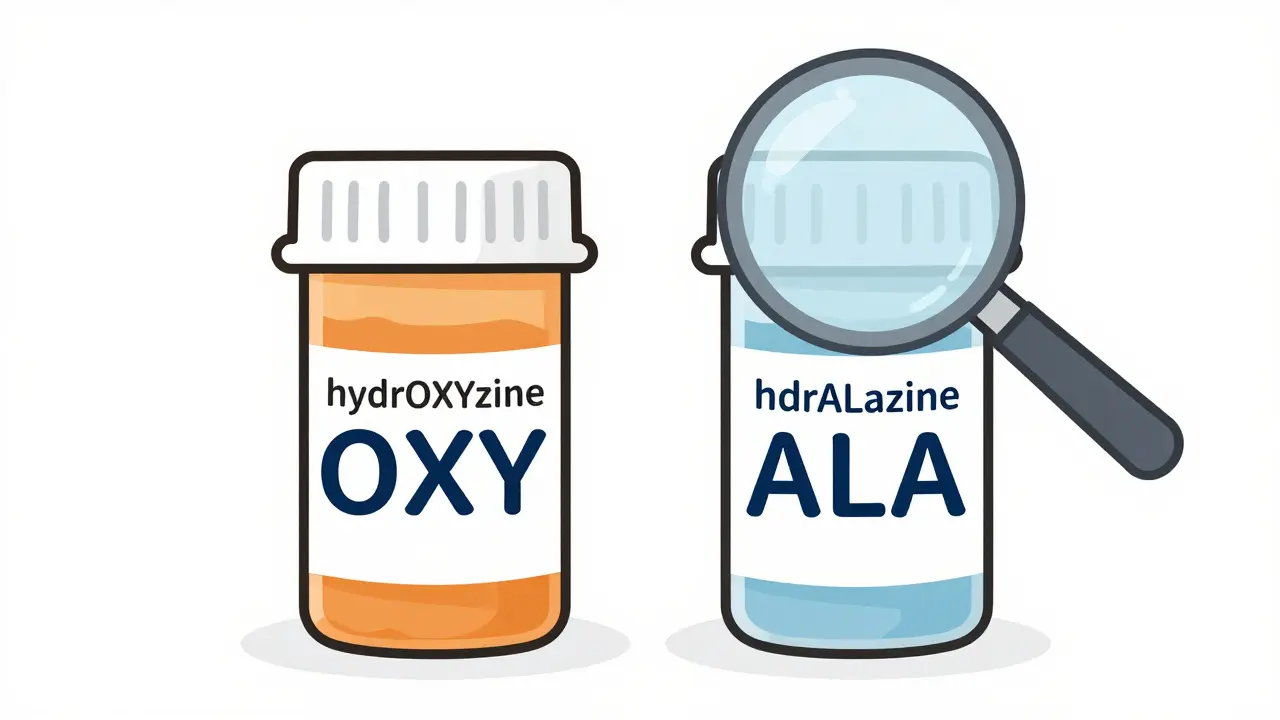
Look-alike and sound-alike (LASA) drug names share enough letters or sounds to trick the brain. For example: hydrOXYzine and hydrALAzine. One’s for allergies, the other for high blood pressure. Mix them up, and you could give someone a dangerous reaction. Or doXEPamine and doBUTamine - both used in critical care, but with totally different effects. One boosts heart function, the other treats depression. Get them swapped, and someone’s heart could stop.
Research shows that when two drug names share 60-80% of their letters, confusion spikes. The FDA and the Institute for Safe Medication Practices (ISMP) have tracked over 3,000 of these risky pairs. Some are obvious - Clonidine and Clonazepam - but others hide in plain sight. Even small changes like a single capitalized letter can make all the difference.
Tall Man Lettering: The Visual Lifesaver
The most common tool to fight this problem is called tall man lettering. It’s simple: you capitalize the parts of the drug name that are different. So instead of writing "hydroxyzine" and "hydralazine," you write hydrOXYzine and hydrALAzine. The capital letters jump out - OXY vs. ALA - making it harder to misread.
The FDA started pushing this method in 2001 after too many patients were hurt by name mix-ups. Today, it’s required in most U.S. hospitals and pharmacies. Studies show tall man lettering cuts visual errors by about 32%. But here’s the catch: it only works if it’s done right. If one system uses it and another doesn’t, you’re back to square one.
For example, a nurse might see hydrOXYzine on the computer screen but then grab a vial labeled "hydroxyzine" in all lowercase from the shelf. That mismatch is exactly how errors slip through. The Joint Commission now requires that tall man lettering be used consistently across all systems - EHRs, automated dispensing cabinets, and printed labels.
It’s Not Just About Letters - Sound Matters Too
Some drug names don’t look similar, but they sound almost identical when spoken. Think Velcade (used for myeloma) and Valtrex (for herpes). Say them out loud - they’re easy to confuse over a phone call or in a busy ward. That’s why the FDA doesn’t just look at spelling. They use advanced algorithms like ALINE to analyze how names sound.
ALINE is 37% better than older tools at spotting sound-alike pairs. It’s built into the drug approval process now. Since 2018, every new drug must pass this test before it hits the market. Between 2018 and 2023, 17 drug names were blocked because they were too similar to existing ones. That’s prevention, not cleanup.
But even with smart tech, human error still happens. A pharmacist hears "valacyclovir" and writes "valganciclovir" - because they sound the same. That’s why the FDA now recommends adding the purpose of treatment on labels. Instead of just "Valtrex," you see "Valtrex - for herpes." That extra detail acts like a safety net.

Technology Can Help - But Only If It’s Set Up Right
Electronic health records (EHRs) are supposed to make things safer. But if they’re poorly configured, they can make errors worse. Here’s what works:
- Minimum 5-character search: If you type "val" into the system, it shouldn’t show 15 drugs. Requiring 5+ letters cuts down confusing lists by 68%.
- No consecutive names: EHRs must never show Hydroxyzine and Hydralazine right next to each other in dropdown menus. That rule alone reduces selection errors by 41%.
- Color coding: Some hospitals use light blue for blood pressure drugs, red for sedatives. When paired with tall man lettering, error rates drop another 15%.
But here’s the problem: not all systems are built the same. A pharmacy might use TML, but the hospital’s EHR doesn’t. A nurse switches between screens and gets confused. A 2023 Reddit thread from pharmacists showed that 63% still see LASA errors - even with TML in place - because of inconsistent tech.
Barcode Scanning: The Gold Standard
Nothing beats barcode scanning for catching errors. When a nurse scans a patient’s wristband and the medication’s barcode before giving a dose, the system checks: Is this the right drug? Right dose? Right patient? Right time?
Studies show barcode scanning prevents 89% of medication errors at the point of administration. That’s huge. But it’s expensive. Hospitals spend an average of $153,000 to install the system. Many smaller clinics can’t afford it.
Still, the payoff is clear. At Johns Hopkins Hospital, combining barcode scanning with tall man lettering and purpose-of-treatment labels cut LASA errors by 67% over two years. That’s not just a number - it’s 67 fewer patients harmed.
What You Can Do: A 3-Step Check
You don’t need fancy tech to stay safe. Here’s a simple, proven method used by top hospitals:
- Read the full label - every time. Don’t just glance at the first few letters. Look at the entire name, the strength, the manufacturer.
- Double-check with someone else - even if you’re confident. Have a colleague verify the drug before it leaves the pharmacy or goes to the patient.
- Read it again at the bedside - before you give it. This final check catches 52% of errors that slipped through earlier.
This isn’t bureaucracy. It’s survival. A 2021 study at UCSF Medical Center found that when staff spent just 2-3 extra minutes per high-risk drug, verification accuracy jumped from 82% to 97%.
Why Handwritten Prescriptions Are Still Dangerous
Even in 2025, some doctors still write prescriptions by hand. And that’s a major risk. A sloppy "c" can look like a "d." A quick "u" can look like an "n."
One nurse reported seeing a handwritten order for "Lortab" - but the doctor meant "Lotronex." One letter. Different drug. Different risk. The patient was lucky - the pharmacist caught it.
According to a 2023 survey, 41% of LASA errors still come from handwritten orders. That’s why e-prescribing is mandatory in most states. If you’re still seeing paper scripts, push for digital. It’s not just convenient - it’s life-saving.
What’s Changing in 2025
The FDA just added 12 more drug pairs to its official tall man lettering list in late 2023. That brings the total to 35 high-risk pairs that all U.S. healthcare systems must now display with proper capitalization. By December 2024, every EHR, pharmacy label, and automated cabinet must comply.
Also new: AI tools like Google Health’s Med-PaLM 2 can now predict LASA confusion with 89% accuracy. They’re not replacing humans - they’re helping them. These tools flag risky names before they even get into the system.
The ISMP is pushing for full TML adoption across all settings by 2026. That includes nursing homes, clinics, and even mail-order pharmacies. The goal? Cut LASA errors in half by 2025.
Final Thought: Don’t Rely on Tech Alone
Tall man lettering, barcodes, and AI are powerful. But they’re not magic. Dr. David Bates from Brigham and Women’s Hospital put it best: "Overreliance on visual differentiation creates false security."
Errors still happen - even with TML - when people are rushed, distracted, or trained poorly. The best defense is a habit: slow down, read the whole label, verify with a second person, and never assume.
Medication safety isn’t about the latest software. It’s about the person holding the bottle. And that person? They need to be awake, aware, and always checking.
What are look-alike and sound-alike (LASA) drug names?
Look-alike and sound-alike (LASA) drug names are medications that have similar spelling or pronunciation, making them easy to confuse. Examples include hydrOXYzine and hydrALAzine, or doXEPamine and doBUTamine. These mix-ups can lead to serious or fatal medication errors if the wrong drug is given.
What is tall man lettering?
Tall man lettering is a visual safety technique where key differentiating letters in similar drug names are capitalized to make them stand out. For example, "hydrOXYzine" and "hydrALAzine" use uppercase letters to highlight the differences (OXY vs. ALA). This method is recommended by the FDA and is now required in most U.S. healthcare systems to reduce confusion.
How effective is tall man lettering at preventing errors?
Tall man lettering alone reduces visual confusion errors by about 32%. When combined with color coding and purpose-of-treatment labels, effectiveness rises to 59%. However, its success depends on consistent use across all systems - EHRs, labels, and dispensing machines.
Why are handwritten prescriptions still a problem?
Handwritten prescriptions are prone to misinterpretation because letters can be unclear - a "c" may look like a "d," or a "u" like an "n." About 41% of LASA errors still come from handwritten orders. Digital prescribing eliminates this risk by standardizing drug names and reducing ambiguity.
What’s the best way to prevent LASA errors?
The most effective approach combines multiple layers: tall man lettering, barcode scanning, purpose-of-treatment labels, and a three-step verification process. Always read the full label, have someone else confirm the drug, and read it again before giving it to the patient. This reduces errors by over 50%.
Are there new technologies helping prevent these errors?
Yes. AI tools like Google Health’s Med-PaLM 2 can predict drug name confusion with 89% accuracy. Smartphone apps with computer vision can scan vials to detect look-alike packaging. The FDA now requires all new drug names to be tested using orthographic (BI-SIM) and phonetic (ALINE) algorithms before approval. These tools are improving safety, but they work best alongside human checks.




Shreyash Gupta
December 27, 2025 AT 04:59Okay but let’s be real - tall man lettering is just a band-aid. I’ve seen EHRs where "hydrOXYzine" shows up as "HYDROXYZINE" because someone "optimized" the font. Then it’s just a block of ALL CAPS. What’s the point? 😅
Zina Constantin
December 28, 2025 AT 23:03Actually, the FDA’s 2023 update to the tall man lettering list was a huge win - 35 high-risk pairs now standardized across all systems. This isn’t just semantics; it’s structural safety. Consistency saves lives. And yes, I checked the Federal Register. 📜
Dan Alatepe
December 29, 2025 AT 08:32Bro. I work in a rural clinic. We don’t have barcode scanners. We don’t even have consistent TML on our printed labels. Last week, someone almost got doXEPamine instead of doBUTamine because the printer faded the "X" into an "O". I had to yell at the IT guy for three hours. This isn’t a policy issue - it’s a survival issue. 😭
Angela Spagnolo
December 30, 2025 AT 17:37I… I just wanted to say… that the three-step check… it’s so simple… but so powerful… I’ve seen it work… in my unit… and it’s… it’s not glamorous… but… it’s everything… :)
Jay Ara
December 31, 2025 AT 06:05man i used to work in a pharmacy and we had this one guy who would always say "read the whole label" like it was a mantra. i thought he was being extra but then he caught a mixup between clonidine and clonazepam because he saw the "dine" vs "zepam". dude saved a life. never underestimate the slow read.
Michael Bond
January 1, 2026 AT 20:43Barcode scanning works. End of story.
Kuldipsinh Rathod
January 3, 2026 AT 07:36my cousin is a nurse in mumbai and she told me they use color-coded stickers on meds because they can't afford e-prescribing. blue for heart, red for sedatives. she says it's not perfect but it's better than nothing. kinda wild that tech gaps are filled with duct tape and sticky notes.
SHAKTI BHARDWAJ
January 5, 2026 AT 00:23YOU ALL ARE JUST TALKING ABOUT LETTERS AND COLORS BUT NO ONE IS TALKING ABOUT THE FACT THAT DOCTORS STILL WRITE PRESCRIPTIONS LIKE THEY'RE IN 1998??!?!? I SAW A "LORTAB" WRITTEN LIKE A CROOKED "L" AND THE PHARMACIST THOUGHT IT WAS "LOTRONEX" AND THE PATIENT ALMOST DIED BECAUSE SOMEONE COULDN'T HOLD A PEN RIGHT!! WHY IS THIS STILL HAPPENING?? I'M SO MAD I COULD SCREAM!!