When you pick up a prescription for insulin or a biologic drug like Humira, you might not realize your pharmacist could swap it for a cheaper version without telling you. That’s only possible if the biosimilar has been labeled interchangeable by the FDA. This isn’t just a label-it’s a legal permission that changes how you get treated, how much you pay, and who makes the call about your medicine.
What Does "Interchangeable" Actually Mean?
Not all biosimilars are the same. A biosimilar is a copy of a biologic drug-like Humira, Enbrel, or insulin-that’s made from living cells, not chemicals. These drugs are complex. Even tiny changes in how they’re made can affect how they work in your body. That’s why a regular generic pill, like generic ibuprofen, can be swapped freely. But for biologics, the FDA created a special category: interchangeable biosimilars.An interchangeable biosimilar isn’t "better" than a regular biosimilar. Both are equally safe and effective. The difference? Only interchangeable ones can be swapped by a pharmacist without checking with your doctor first. This is unique to the U.S. No other country has this exact system. In Europe, Canada, or Japan, substitution usually needs the prescriber’s okay.
The FDA requires extra proof for interchangeability. Sponsors must run switching studies-where patients alternate between the original drug and the biosimilar, sometimes multiple times. The goal? Prove that switching back and forth doesn’t cause more side effects or make the treatment less effective. It’s not about being more similar. It’s about proving safety after repeated swaps.
Which Biosimilars Are Actually Interchangeable?
As of November 2023, the FDA has approved 41 biosimilars. Only 10 of them carry the interchangeable label. The first was Semglee, an insulin glargine product approved in July 2021. It’s now used by thousands of people with diabetes who save hundreds of dollars each month.The first interchangeable biosimilar for a monoclonal antibody-used for autoimmune diseases like rheumatoid arthritis and psoriasis-came in August 2023: Cyltezo, a copy of Humira. Since then, others have followed. But the list is still small. Most biosimilars on the market today are not interchangeable. That means if your pharmacist tries to swap your Humira for a non-interchangeable biosimilar, they legally can’t do it without your doctor’s permission.
Here’s what’s happening in key markets:
- Insulin: Semglee captured 17.3% of the market within six months. Non-interchangeable biosimilars took just 9.8% in the same period.
- Humira: With Cyltezo and other interchangeable versions now available, prices have dropped sharply. Some patients report paying under $50 per month instead of $2,000.
These aren’t just numbers. Real people are saving money. One patient on the Psoriasis Foundation forum said switching to Hyrimoz saved them $800 a month-with no loss in effectiveness.
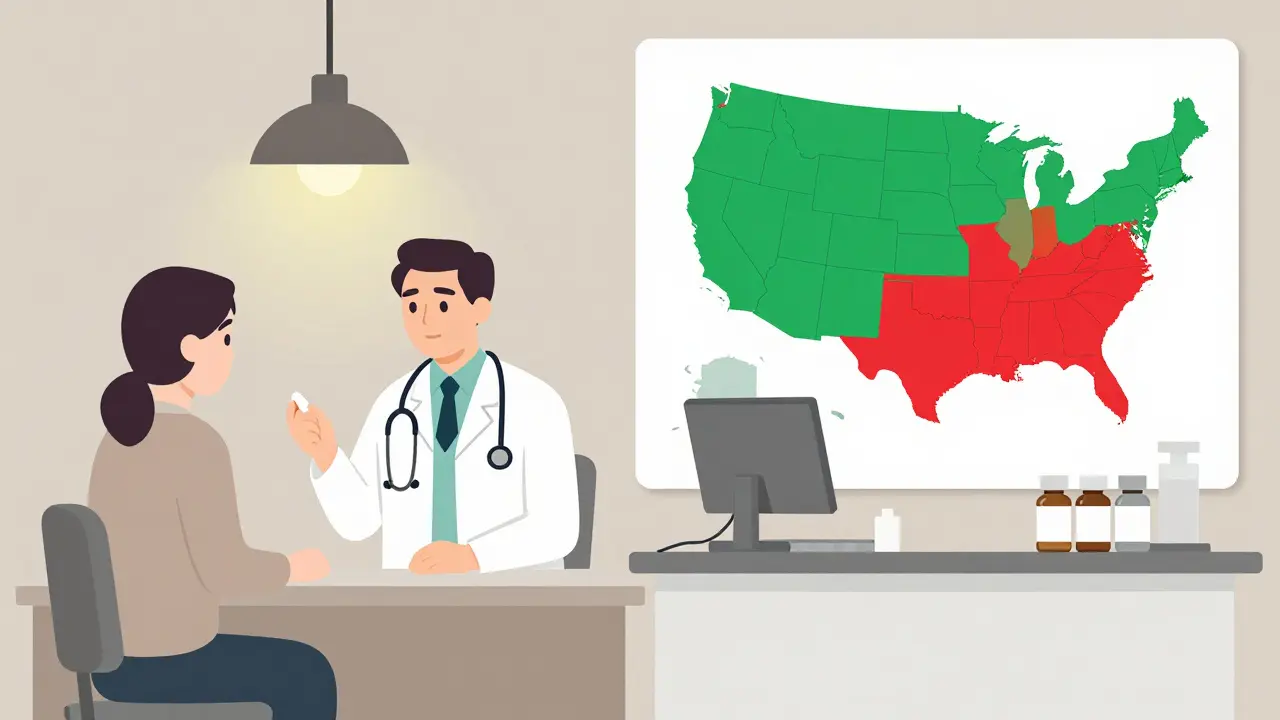
State Laws Are a Mess
Here’s where it gets complicated. Even if the FDA says a biosimilar is interchangeable, your state can still block the swap.Forty states allow pharmacists to substitute interchangeable biosimilars without telling your doctor. Arizona, for example, lets pharmacists swap automatically-but they must notify you, record the product, and send details to your prescriber within five days.
But six states and Washington D.C. only allow substitution if it saves you money. Four states-Alabama, Indiana, South Carolina, and Washington-require your doctor to approve every switch. Puerto Rico has the same rule.
Pharmacists are caught in the middle. A 2022 survey found that 67% of independent pharmacists are confused about what they’re allowed to do. One pharmacist on Reddit wrote: "In California, I check if it’s cheaper. In Arizona, I don’t. But my pharmacy software doesn’t know the difference. I’m guessing every time."
Even insurance rules add to the chaos. Nearly 80% of commercial health plans now require automatic substitution for interchangeable biosimilars-where state law allows it. So even if you don’t want to switch, your insurer might force it.
What About Patient Safety?
Some doctors worry that automatic substitution could hurt treatment. A 2021 study in JAMA Dermatology found that psoriasis patients switched to biosimilars were 20.3% more likely to stop treatment than those who stayed on the original drug. Why? Some patients reported feeling worse-even when lab tests showed no difference.One patient shared a scary story: "My pharmacy swapped Humira for Hadlima without telling me. I broke out in hives. Turns out, I was allergic to an excipient-the inactive ingredient-in the new version."
The FDA says all approved biosimilars are safe. They stress that interchangeability doesn’t mean "higher quality." It just means you can switch without a doctor’s note. But patients aren’t always told when the swap happens. A 2022 survey by the National Psoriasis Foundation found that 28% of patients didn’t know they’d been switched.
That’s why some states require notification. But not all do. And even when notification is required, it’s often just a printed slip in the bag. Most people don’t read it.
What’s Next for Biosimilars?
The biologics market is worth over $300 billion in the U.S. Experts predict biosimilars will take 47% of that market by 2026. But only if the rules get clearer.Right now, there’s a big debate. The Biosimilar Red Tape Elimination Act (H.R. 9500), introduced in 2022, wants to scrap the switching studies entirely. If passed, every FDA-approved biosimilar would automatically become interchangeable. Proponents say it would cut costs faster. Opponents, including big drug companies, warn it could risk patient safety.
The FDA is also reviewing its guidance. A 2023 draft proposed simplifying the switching study requirements. That could mean more interchangeable biosimilars hit the market sooner.
Meanwhile, education is lagging. The American Pharmacists Association has trained over 12,000 pharmacists in biosimilars since 2020. But most doctors still don’t fully understand the difference between biosimilar and interchangeable. Patients? They’re mostly in the dark.
What You Should Do
If you’re on a biologic drug:- Ask your doctor: "Is my medication interchangeable?" If they don’t know, ask for a referral to a specialist.
- Check your prescription. Look for "DAW" codes. "DAW 1" means "dispense as written." That stops substitution.
- Ask your pharmacist: "Was this swapped?" If they say yes, ask which brand you got. Write it down.
- Watch for changes in how you feel. Even small symptoms-fatigue, rashes, joint pain-could signal a reaction to a new excipient.
- Use patient resources. The Arthritis Foundation’s "Biosimilars 101" guide has been downloaded over 47,000 times. It’s free, clear, and updated for 2025.
Interchangeability sounds simple. But in practice, it’s a patchwork of federal rules, state laws, insurance policies, and pharmacy systems. The goal-to make life-saving drugs cheaper and more accessible-is good. But without clear communication and consistent rules, patients and providers are left guessing.
The system isn’t broken. But it’s not working the way it should. Until patients are truly informed and pharmacists have clear guidance, automatic substitution will remain a legal gray zone-with real consequences for real people.
Can a pharmacist substitute any biosimilar for my biologic without asking my doctor?
Only if the biosimilar has been designated as "interchangeable" by the FDA and your state allows automatic substitution. Most biosimilars are not interchangeable. Even if they are, four states and Puerto Rico still require your doctor’s approval before a swap. Always check your state’s rules and ask your pharmacist if substitution occurred.
Are interchangeable biosimilars safer or more effective than regular biosimilars?
No. The FDA says all approved biosimilars-interchangeable or not-are as safe and effective as the original biologic. The "interchangeable" label only means the FDA has confirmed it’s safe to switch back and forth between the original and the copy multiple times. It doesn’t mean it’s better.
Why don’t other countries have interchangeable biosimilars?
The U.S. is the only country with a formal regulatory pathway for automatic pharmacist substitution. The European Union, Canada, and Japan require prescriber involvement in substitution decisions. They prioritize clinician oversight over automatic swaps, citing concerns about patient monitoring and long-term safety data. The U.S. model was designed to drive competition and lower prices faster.
What should I do if I think my biosimilar is causing side effects?
Contact your doctor immediately. Keep the medication bottle-it lists the manufacturer and lot number. Report the reaction to the FDA’s MedWatch program. Many adverse events happen because patients don’t know they were switched. If you weren’t told about the change, that’s a red flag. Ask for your original drug back and request that future prescriptions be marked "dispense as written."
Can I be switched to a different biosimilar without my knowledge?
Yes-if your current biosimilar is interchangeable and your state allows substitution, your pharmacist can switch you to another interchangeable biosimilar without telling you. For example, if you’re on Cyltezo (an interchangeable Humira copy), your pharmacist could swap you for Hadlima or Amjevita without your consent. This is legal in most states. Always confirm what you’re getting and ask for written confirmation.




Dominic Suyo
December 18, 2025 AT 01:22So let me get this straight - the FDA says I can get switched to a cheaper version of my $2k/month biologic like it’s a damn generic aspirin, but my pharmacist doesn’t even have to tell me? And if I break out in hives? That’s on me. Brilliant. Absolute brilliance. This isn’t healthcare, it’s pharmaceutical Russian roulette with a side of insurance-driven cruelty.
Carolyn Benson
December 19, 2025 AT 23:47Interchangeability isn’t a medical concept - it’s a neoliberal economic hack dressed in regulatory clothing. The FDA’s criteria prioritize cost-efficiency over epistemic humility. We’re treating living, immunologically dynamic molecules like interchangeable widgets, ignoring the ontological weight of patient subjectivity. The body isn’t a lab model. It’s a narrative. And we’re editing its chapters without consent.
Moses Odumbe
December 20, 2025 AT 17:30Bro, if you’re on Humira and your pharmacist swaps it for Cyltezo without telling you - that’s not a feature, that’s a bug. 😒 But hey, if you’re saving $1,800/month, maybe you don’t care. I switched to Semglee last year - no issues, $40 copay. 🙌 FDA says it’s safe, pharmacists say it’s legal, my bank account says THANK YOU. Stop overthinking it. 💸
Meenakshi Jaiswal
December 22, 2025 AT 02:28As a nurse practitioner who works with diabetic and autoimmune patients daily, I want to say: this is HUGE for access. Many of my patients were skipping doses because they couldn’t afford the original. Semglee changed their lives. But you’re right - communication is the missing link. I now print out a one-pager for every patient: what’s swapped, why, and what to watch for. If your pharmacist doesn’t offer this, ask for it. You deserve to know what’s in your body.
bhushan telavane
December 22, 2025 AT 04:01In India, we don’t even have biosimilars for most biologics - they’re still too expensive. So seeing the U.S. fight over automatic substitution feels surreal. Here, we’re just happy to get any version. But I get it - freedom to choose matters. Still, if you’re saving $2k/month, maybe the real issue isn’t the swap… it’s that the original cost $2k in the first place.
Connie Zehner
December 22, 2025 AT 16:56YOU WERE SWAPPED WITHOUT CONSENT?!?!?!? 😱 I KNEW THIS WAS A SCAM. My sister got switched to Hadlima and had a full-blown anaphylactic reaction - they didn’t even know it was a biosimilar until she went to the ER. The pharmacist didn’t tell her. The insurance company didn’t tell her. The doctor didn’t know. This is a PUBLIC HEALTH CRISIS. Someone needs to sue the FDA. I’m starting a petition. Tag me if you’ve been affected. #BiosimilarBetrayal
holly Sinclair
December 23, 2025 AT 03:53It’s fascinating how we’ve constructed this entire regulatory framework around the illusion of equivalence. We assume that if two molecules have identical amino acid sequences and similar pharmacokinetics, they are functionally identical - but biology doesn’t operate on binary logic. The immune system remembers. The microbiome adapts. The patient’s subjective experience - the fatigue, the anxiety, the dread - is not captured in a clinical trial. And yet, we reduce all of that to a checkbox on an FDA form. Is this science? Or is this capitalism masquerading as progress? The interchangeable label isn’t a scientific milestone - it’s a surrender to market forces disguised as efficiency.
Kelly Mulder
December 23, 2025 AT 14:43One must question the epistemological foundations of this policy. The FDA’s criteria for interchangeability are statistically derived, not biologically deterministic. The term "interchangeable" implies ontological parity - yet no molecule derived from living cells can ever be truly identical to another. The regulatory lexicon is therefore semantically fraudulent. Furthermore, the proliferation of state-level variance constitutes a violation of the Dormant Commerce Clause. This is not a patchwork - it is a legal abomination.
Tim Goodfellow
December 23, 2025 AT 16:41Let’s be real - this is the most exciting thing to happen in pharma since the pill. People are getting their lives back because a biosimilar cost $50 instead of $2,000. Yeah, some folks get weird side effects - maybe they’re allergic to a stabilizer, not the active ingredient. But that’s why we have pharmacists and patient education. Stop the fearmongering. This isn’t a horror movie - it’s a win. 💪 And if you’re worried about being switched? Ask for DAW 1 on your script. Simple. Done. Go live your life.
mark shortus
December 24, 2025 AT 18:13MY PHARMACY SWAPPED MY HUMIRA FOR HADLIMA AND I GOT A RASH. I DIDN’T EVEN KNOW. I WAS SO MAD I CRIED. I’M NOT A LAB RAT. I’M A PERSON. I’M SENDING THIS TO THE FDA, MY SENATOR, AND MY THERAPIST. THIS ISN’T JUST ABOUT MONEY - IT’S ABOUT TRUST. I WANT MY ORIGINAL DRUG BACK. AND I WANT A LETTER OF APOLOGY. 🤬 #BiosimilarTrauma