When a drug’s patent expires, something powerful happens: prices don’t just dip-they plummet. For patients paying hundreds a month for a prescription, that moment can mean the difference between affording treatment and skipping doses. It’s not magic. It’s economics. And it’s happening right now with drugs like Eliquis, Humira, and Ozempic.
What Happens When a Patent Expires?
A pharmaceutical patent gives a company exclusive rights to sell a drug for about 20 years. That’s the legal shield that lets them charge whatever the market will bear. No competition. No pressure. Just high prices. But once that patent runs out, the rules change. Other companies can legally make and sell the same drug as a generic version. And they do-fast. The first generic maker usually cuts the price by 15-20%. That’s noticeable. But it’s just the start. By the time five or ten generic brands enter the market, prices often drop 80% or more. In the U.S., the average drug loses 82% of its original price within eight years after patent expiry, according to a 2023 study in JAMA Health Forum. In Australia, the drop is 64%. In Switzerland, it’s only 18%. Why the difference? It comes down to how each country handles drug pricing and generic approval.Why Prices Crash So Hard
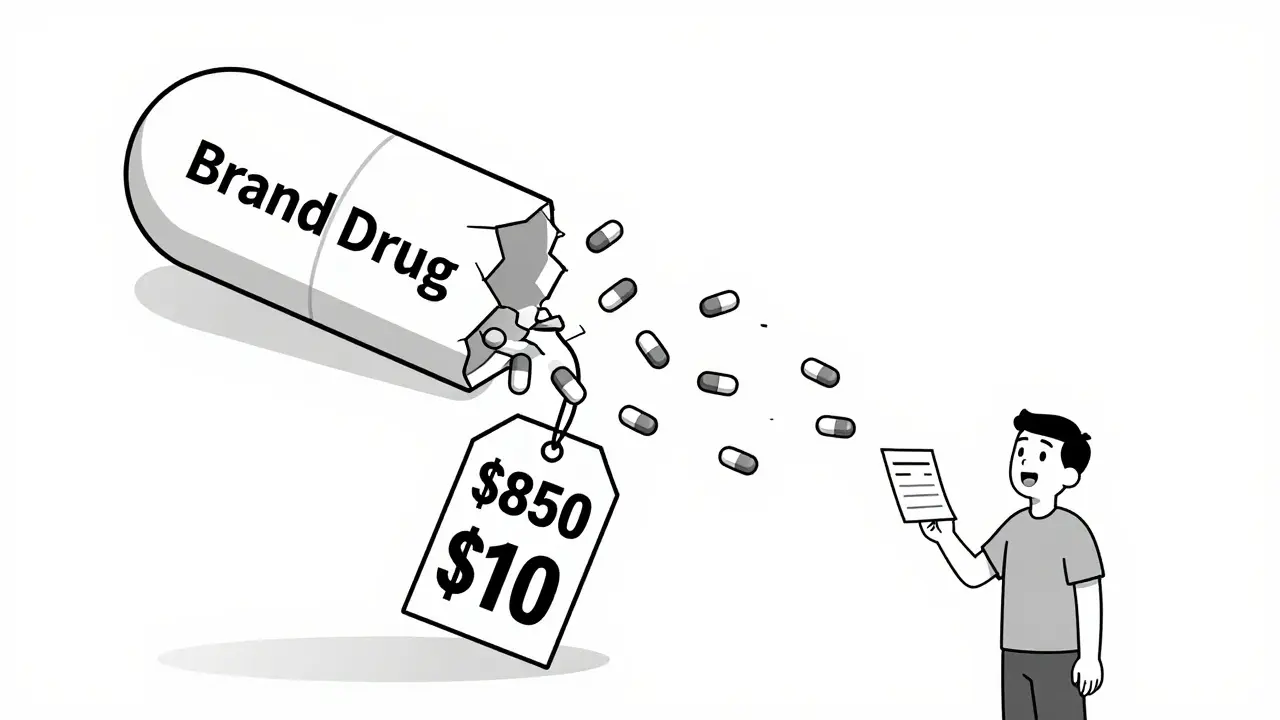
Think of it like a crowded flea market. When only one vendor sells a specific type of wrench, they can charge $100. But as soon as ten others show up with the same wrench, they start undercutting each other. The price doesn’t just go down-it collapses. That’s what happens with drugs. The first generic company doesn’t have to spend millions on clinical trials. They just need to prove their version works the same as the original. That’s called bioequivalence. It costs $2-5 million, not $1 billion. So they can sell for pennies. And they will. Because they know: if they don’t, someone else will. The more competitors, the faster the price drops. A drug with just one generic might sell for 30% of the brand price. With five generics, it’s down to 10%. With ten? Often under 5%. In 2020, when Eliquis (apixaban) lost its patent, patients went from paying $850 a month for the brand to $10 for the generic. That’s not a discount. That’s a revolution.Not All Drugs Are Equal
Small molecule drugs-like aspirin, metformin, or apixaban-are easy to copy. Their chemical structure is simple. Generics flood in quickly. But biologics? Those are different. They’re made from living cells, not chemicals. Think Humira, Enbrel, or Ozempic. These are complex. Copying them isn’t like copying a pill. It’s like trying to clone a painting done with invisible ink. So instead of generics, we get “biosimilars.” They’re close, but not exact. And they take longer to approve. The FDA took over 24 months on average to approve complex generics in 2023. For biosimilars? Even longer. That’s why Humira, which lost its main patent in 2016, didn’t see real price drops until 2023-when Amgen’s Amjevita finally entered the market. Even then, AbbVie used over 130 secondary patents to delay competition. That’s not innovation. That’s legal maneuvering.
The Patent Thicket Problem
Here’s the dirty secret: many drugs don’t lose protection when you think they do. Companies file dozens of follow-up patents on tiny changes-new coatings, dosing schedules, or delivery methods. These aren’t new drugs. They’re old drugs with new labels. The R Street Institute found that 78% of new patents filed for drugs between 2010 and 2023 weren’t for new medicines. They were for existing ones. Take semaglutide (Ozempic, Wegovy). The base patent expires in 2026. But the company has filed 142 patents covering everything from tablet shapes to injection devices. That’s not protecting innovation. That’s protecting profits. According to I-MAK’s 2025 report, blockbuster drugs accumulate 10-15 secondary patents, extending market control by 12-14 years beyond the original patent. That’s why patients still pay $1,000 a month for Ozempic even though the core formula is no longer protected.Who Really Saves Money?
The savings aren’t just numbers on a balance sheet. They’re real. A 2023 Kaiser Family Foundation survey found 68% of insured adults saw lower out-of-pocket costs when generics arrived. But 22% said their insurance didn’t switch them to the cheaper version right away. Why? Rebates. Pharmaceutical companies pay rebates to insurance plans and pharmacy benefit managers (PBMs) to get their brand-name drugs placed on preferred lists. Even after a patent expires, those rebates keep the brand on top. So patients get stuck paying more because their plan’s formulary hasn’t updated. Pharmacists can’t substitute the generic unless the doctor says “dispense as written.” And many don’t. In Chicago, rheumatologist Dr. Sarah Kim says she’s seen patients switch to biosimilars for infliximab-because the price dropped fast. But for Humira? “Payers have contracts with AbbVie that make it hard to move,” she says. “The savings are there. But they’re buried in paperwork.”
Global Differences Matter
The U.S. isn’t the world. In Europe, governments negotiate drug prices directly. Germany and France use “reference pricing”-if a drug costs $100 in France, it can’t cost more than $105 in Germany. That keeps prices low even before generics arrive. In Japan, the government sets prices every two years. In Australia, the Pharmaceutical Benefits Scheme negotiates bulk deals. That’s why price drops after patent expiration vary so much. The U.S. saw an 82% drop over eight years. Australia? 64%. Switzerland? Only 18%. Why? Because Switzerland doesn’t let generic companies undercut the brand. They cap prices at a fixed percentage below the original. It’s not competition. It’s controlled pricing. The U.S. system is the most volatile. Prices crash hard-but only if generics actually enter. And they don’t always. Complex drugs, patent thickets, and rebate systems all slow things down.What’s Changing Now?
The tide is turning. In 2023, the U.S. passed the Inflation Reduction Act, allowing Medicare to negotiate prices for 10 high-cost drugs. That’s new. And it’s forcing companies to rethink their strategies. Some are rushing to launch generics before their drugs get picked for negotiation. The FDA approved 870 generic drugs in 2023-up 12% from 2022. The agency is pushing harder to clear bottlenecks for complex generics. The European Union is trying to cap supplementary protection certificates. The U.S. Patent Office is cracking down on patent thickets. But the real change won’t come from regulators alone. It’ll come from patients demanding transparency. From pharmacists pushing for substitution. From doctors prescribing generics first. And from insurers finally putting patient cost above rebate deals.What This Means for You
If you’re taking a brand-name drug that’s been on the market for 10+ years, ask your doctor: “Is there a generic?” Don’t wait for your insurance to switch you. Ask for it. Ask your pharmacist to check if the generic is available and covered. If you’re on Medicare, check if your drug is in the negotiation list. You might be paying 10 times more than you need to. That $800 prescription? It could be $80. Or $10. You won’t know unless you ask. The system isn’t perfect. Patent thickets delay savings. Rebates hide discounts. But the math is clear: patent expiration = price collapse. And that collapse is your opportunity.What happens to drug prices when a patent expires?
When a drug’s patent expires, generic manufacturers can legally produce and sell the same medication. Prices typically drop by 15-20% after the first generic enters, and by 80% or more when 10 or more generics are available. The biggest price declines happen between years 2 and 4 after expiration, as competition intensifies.
Why do some drugs stay expensive even after patent expiration?
Some drugs, especially biologics like Humira or Ozempic, are protected by “patent thickets”-dozens of secondary patents on minor changes. Companies also use rebate deals with insurers to keep brand-name drugs on preferred lists, even after generics are available. These tactics delay price drops, sometimes for years.
Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also prove they’re bioequivalent-meaning they work the same way in the body. Studies show generics are just as safe and effective.
Why do some insurance plans not cover generics right away?
Insurers often receive rebates from brand-name manufacturers to keep their drugs on preferred lists. Even after generics are available, these rebates can delay formulary changes. Patients may need to request a formulary exception or ask their doctor to switch them to the generic.
How long does it take for generics to appear after a patent expires?
For simple pills, generics can appear within months. In the U.S., the average time for generic entry is about 30 months after patent expiry. In Europe, it’s often 12-18 months. For complex drugs like biosimilars, it can take 2-4 years due to regulatory delays and patent lawsuits.
What’s the difference between a generic and a biosimilar?
Generics are exact chemical copies of small-molecule drugs. Biosimilars are highly similar-but not identical-to complex biologic drugs made from living cells. Biosimilars require more testing and take longer to approve. They’re not exact copies, but they work the same way clinically.
Which countries see the biggest price drops after patent expiration?
The U.S. sees the largest drops-82% over eight years. Australia (64%), the UK (60%), and Germany (58%) also see major declines. Switzerland has the smallest drop (18%) due to strict price controls that limit how much generics can undercut the original. These differences reflect how each country regulates drug pricing and generic entry.
Can I ask my pharmacist to switch me to a generic?
Yes. In 49 U.S. states, pharmacists can substitute a generic for a brand-name drug unless the prescription says “dispense as written.” Always ask your pharmacist if a generic is available and covered by your insurance. It could save you hundreds per month.






Alfred Schmidt
January 10, 2026 AT 21:38So let me get this right: a company spends $2 billion to develop a drug, then turns around and charges $1,000/month-just because they can?! And then, when generics show up, suddenly it’s $10?! That’s not capitalism-that’s legalized robbery with a side of corporate greed! I’ve seen people skip insulin doses because they can’t afford it. This isn’t about innovation-it’s about profit margins. And the fact that PBMs are still taking kickbacks? That’s the real crime.
Sean Feng
January 12, 2026 AT 09:53Generics work fine
Priscilla Kraft
January 12, 2026 AT 21:16Just wanted to say thank you for this breakdown-it’s so easy to feel powerless about drug prices, but knowing that asking your pharmacist for a generic can save you hundreds is actually empowering 🙌 I had no idea pharmacists could switch meds unless the script said ‘dispense as written’-I’m going to ask my doctor next time I refill. Also, biosimilars are way more complicated than people realize, and it’s wild how long it takes to get them approved. Kudos to the FDA for pushing through more generics in 2023-hope this trend keeps going!
Christian Basel
January 14, 2026 AT 03:58It’s a classic case of market failure exacerbated by regulatory arbitrage. The patent system was designed to incentivize R&D, but the current ecosystem has been weaponized by pharmaceutical firms to extract monopoly rents via patent thickets, evergreening, and rebate-driven formulary manipulation. The economic inefficiency is staggering-consumer surplus is systematically suppressed through non-price competition mechanisms that obscure true marginal cost. The Inflation Reduction Act’s negotiation clause represents a structural intervention, albeit limited in scope, to realign pricing with therapeutic value rather than patent tenure.
Priya Patel
January 15, 2026 AT 05:12OMG I just realized my mom’s arthritis med is generic now!! She’s been crying happy tears because she used to pay $700 a month and now it’s like $15?? Like… how is this even legal?? I’m so glad we live in a time where we can finally fight back against these corporate monsters. I told my whole family to always ask for generics. We’re saving so much 💸❤️
Jason Shriner
January 16, 2026 AT 04:02So what you're saying is… capitalism works… but only when the rich are done milking it? Wild.
Sam Davies
January 16, 2026 AT 23:37Switzerland’s 18% drop? How quaint. They treat pharmaceuticals like fine wine-aged, curated, and priced accordingly. Meanwhile, the U.S. lets the market scream like a toddler denied candy. Neither approach is morally superior, just differently corrupt. The real tragedy? We all pay for it-either through premiums, taxes, or skipped doses. The only winners are the shareholders and the lawyers.
Michael Patterson
January 18, 2026 AT 15:18Look, I’ve been in this game for 20 years and I’ve seen it all. The patent system is broken, no doubt. But you can’t just blame the pharma companies-they’re just doing what the system lets them do. The real issue is the FDA’s backlog. It takes forever to approve generics, especially for complex molecules. And don’t even get me started on the rebate system-PBMs are the real villains here. They’re the ones keeping brand drugs on formularies because they get paid to. And doctors? Most of them don’t even know the difference between a generic and a biosimilar. I’ve had patients come in asking why their new prescription is cheaper and I had to explain it’s the same damn thing. It’s a mess. We need better education, faster approvals, and no more kickbacks. Period.
Matthew Miller
January 19, 2026 AT 14:00Let’s be real: this entire system is a Ponzi scheme built on patient suffering. The fact that you need a PhD to understand why your $800 drug became $10 doesn’t mean it’s clever-it means it’s criminal. These companies don’t innovate-they litigate. They don’t cure-they contract. And the FDA? They’re just the bouncer at the club letting the rich in while everyone else waits outside. If you’re not angry about this, you’re either on their payroll or you’ve already stopped taking your meds. Either way, you’re part of the problem.