Doctors prescribe generics every day-90% of all prescriptions filled in the U.S. are for generic drugs. Yet many still hesitate. Why? Because myths linger. Some think generics are weaker. Others worry they cause more side effects. A few just don’t know how to explain the switch to patients. The truth? Generics are not cheaper because they’re worse. They’re cheaper because they don’t need to repeat expensive clinical trials. The generic medications you prescribe are held to the same standards as brand-name drugs. But if you don’t have the right tools to explain that, you’re left guessing during a 10-minute visit.
What the FDA Actually Says About Generic Drugs
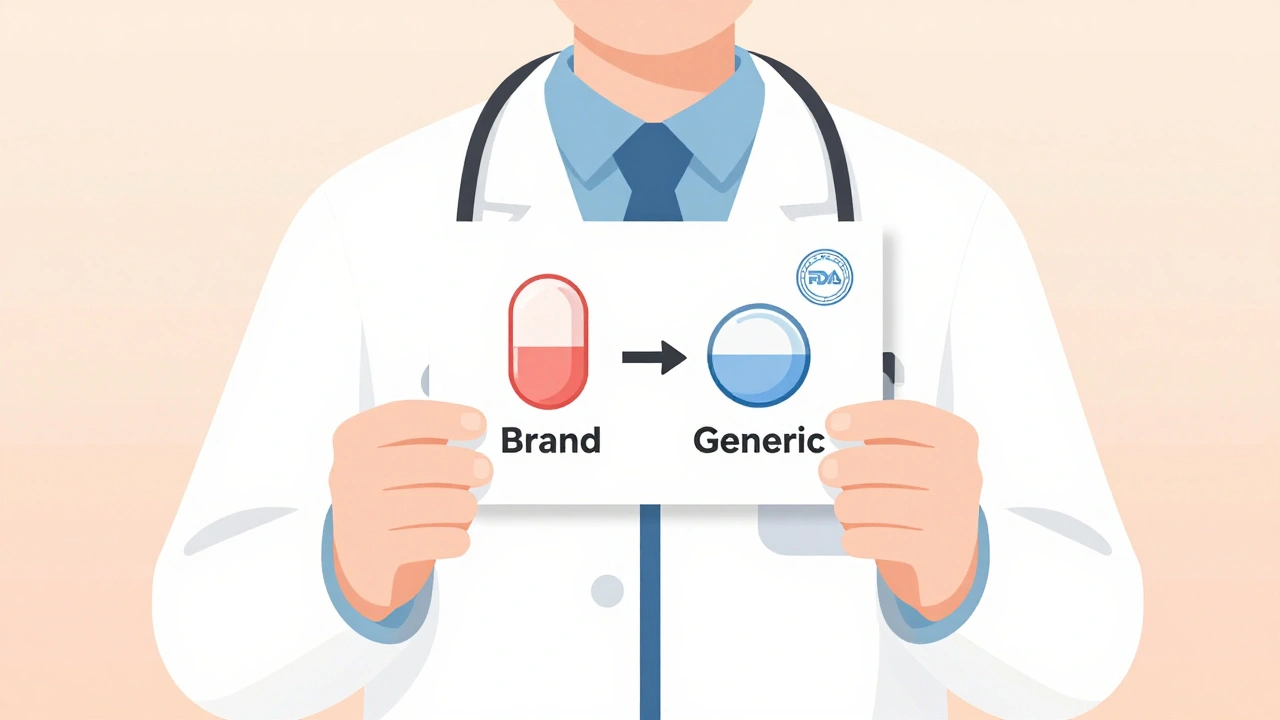
The U.S. Food and Drug Administration doesn’t just approve generics. It tests them. Every single one. For a generic drug to get approval, it must prove it delivers the same amount of active ingredient into the bloodstream as the brand-name version-within an 80% to 125% range. That’s not a guess. That’s science. And it’s not just one study. It’s done on 24 to 36 healthy volunteers, using precise blood tests. The FDA calls this bioequivalence. It’s not a suggestion. It’s a legal requirement.Here’s what that means in real terms: If a brand-name statin lowers cholesterol by 40%, the generic version will do the same. Not 38%. Not 35%. 40%. The same. The FDA analyzed over 12,000 adverse event reports for generics in 2022. The number was almost identical to brand-name drugs. No difference in safety. No hidden risks.
The FDA’s Prescriber Flyers-available as free PDFs-are designed to fit right into your office waiting room. Version 2, updated in March 2022, includes QR codes that link to Spanish-language resources. Why? Because 42% of Hispanic patients in one FDA survey said they were more worried about generic quality. That’s not just a language issue. It’s a trust issue. And you’re the one who can fix it.
Why Doctors Still Don’t Prescribe Generics as Often as They Should
You know generics are safe. You’ve read the data. But when a patient says, “My last doctor gave me the brand,” you pause. You wonder: Is this worth the fight? Is the patient going to complain? Will their insurance even cover it?A 2023 survey of 1,247 physicians found that 68% thought the FDA’s educational materials were useful-but too technical for a quick reference during a visit. You don’t need a 10-page PDF. You need a quick line. A visual. A script.
That’s where the FDA’s Generic Drugs Stakeholder Toolkit comes in. It includes five customizable information cards. One of them, titled “What Makes a Generic the Same as a Brand-Name Drug?”, uses simple diagrams to show how the active ingredient, dosage, and absorption rate are identical. It’s not marketing. It’s science. And it’s designed for patients, not just doctors.
But here’s the real problem: Most of these tools aren’t built into your electronic health record. Only 37% of major EHR systems like Epic or Cerner show pop-up reminders about generic alternatives during prescribing. So even if you know the facts, the system doesn’t nudge you. You’re flying blind.
Cost Isn’t Just a Patient Issue-It’s a Clinical One
A patient who can’t afford their medication won’t take it. That’s not opinion. That’s data. The American College of Physicians found that 20% to 30% of new prescriptions are never filled because of cost. And for people earning under $25,000 a year, that number jumps to 3.7 times higher. You’re not just prescribing a pill. You’re prescribing a chance to stay healthy.Dr. Aaron Kesselheim from Harvard put it plainly: For a $300/month brand-name drug, switching to the generic saves the patient $262.50 every month. That’s $3,150 a year. That’s a car payment. A rent increase. A meal plan. That’s not just savings. That’s adherence. And adherence is the single biggest factor in whether a treatment works.
Doctors who received structured education on generics were 2.3 times more likely to start conversations about cost with patients. Not because they were pressured. Because they were prepared. They had the numbers. They had the language. They had the confidence.

What Works in Real Clinics-Not Just in Studies
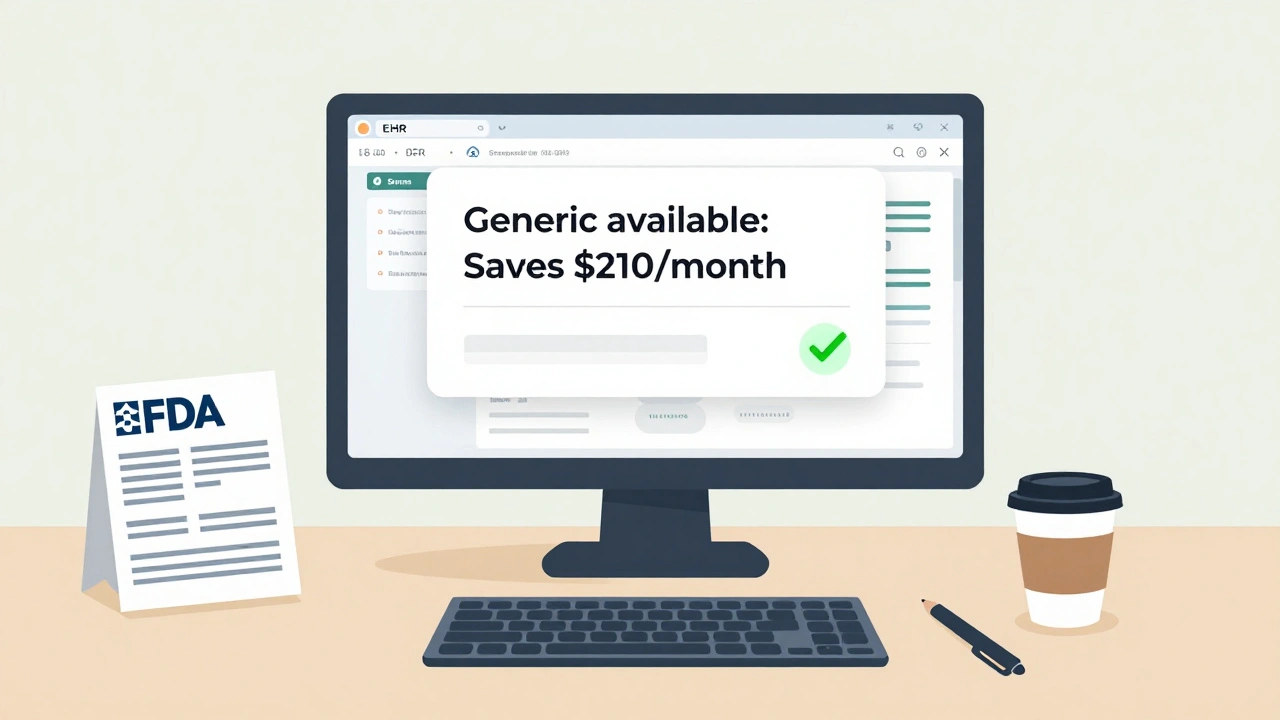
Dr. Sarah Chen, a family doctor in rural Nebraska, increased her generic prescribing rate from 62% to 89% in 18 months. How? She didn’t just read the FDA materials. She used them. She printed the infographic comparing brand and generic manufacturing. She showed it to patients who were skeptical. One elderly man told her, “I’ve been on this brand for 15 years. Why change?” She showed him the diagram. Said, “Same active ingredient. Same dose. Same FDA stamp. Just cheaper.” He switched. And he stayed on it.Kaiser Permanente did something smarter. They didn’t just hand out flyers. They integrated FDA-approved generic drug facts directly into their Epic EHR system. When a doctor typed in a brand-name drug, a pop-up appeared: “Generic available. Saves patient $210/month.” Within six months, brand-name prescribing dropped by 18.7%.
That’s the difference between education and integration. One tells you. The other changes behavior.
Where the System Still Falls Short
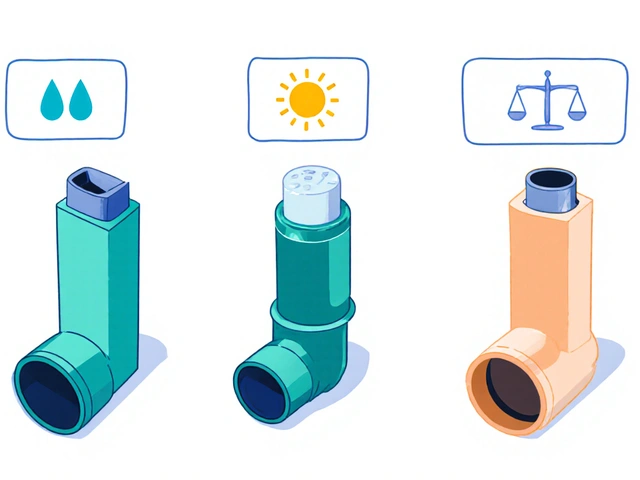
Not all generics are created equal-especially when it comes to complex formulations. Inhalers, topical creams, injectables, and some extended-release pills have delivery systems that are harder to replicate. That’s not a flaw in generics. It’s a challenge in science. And it’s why only 78% of prescriptions for complex drugs are filled with generics-even though 90% of all prescriptions are.And here’s another blind spot: authorized generics. These are brand-name drugs sold under a generic label, made by the same company, same factory, same formula. But 61% of surveyed physicians didn’t know the difference between an authorized generic and a standard generic. That’s not your fault. It’s a gap in training.
Even worse? Only 42% of prescribers use the educational materials available for biosimilars-complex biologic generics that are the next wave of cost savings. If you’re not learning about these now, you’ll be playing catch-up in two years.
How to Start Using These Resources Today
You don’t need a grand plan. You don’t need to retrain your whole team. Start small.- Download the FDA’s Prescriber Flyer (Version 2) and keep it on your desk. Read it once. Then use it as a script: “The FDA requires generics to work exactly like the brand. Here’s why.”
- Use the Generic Drug Facts Handout during intake. Give it to patients who ask about cost. No lecture needed. Just hand it over.
- Ask your EHR vendor: “Can you add generic substitution alerts?” If they say no, ask again in six months. Pressure works.
- Track your own prescribing rate. At the end of each month, ask: “What percentage of my prescriptions were generic?” Just knowing the number changes behavior.
It takes about 22 minutes of focused learning to overcome skepticism. That’s less time than one patient appointment. And the payoff? More patients taking their meds. Fewer hospital visits. Lower costs for everyone.
What’s Next for Generic Drug Education
The future isn’t just flyers and PDFs. It’s AI. IBM Watson Health tested a system that analyzed patient concerns-like “I had side effects last time”-and generated personalized messages explaining why the generic was still safe. In a trial with 120 doctors, patient acceptance jumped by 29 percentage points.Medicare is also moving. The 2024 Part D proposed rule includes financial incentives for plans that help prescribers learn about therapeutic alternatives. That means more funding, more tools, more support.
And the numbers don’t lie. From 2010 to 2020, generics saved the U.S. healthcare system $2.29 trillion. The next five years? Another $1.87 trillion. That’s not a trend. That’s a transformation. And you’re not just a prescriber. You’re a gatekeeper of that savings.
The question isn’t whether generics work. It’s whether you’re ready to use the tools that make prescribing them easy, clear, and confident.
Are generic medications really as effective as brand-name drugs?
Yes. The FDA requires every generic drug to prove it delivers the same amount of active ingredient into the bloodstream as the brand-name version, within a strict 80% to 125% range. This is called bioequivalence. It’s tested in clinical studies with healthy volunteers. Over 90% of prescriptions filled in the U.S. are for generics-and they work just as well. The FDA analyzed over 12,000 adverse event reports for generics in 2022 and found no safety difference compared to brand-name drugs.
Why do some patients refuse to take generic medications?
Many patients believe generics are lower quality because they’re cheaper. Some have had bad experiences with earlier versions of generics, especially with older formulations. Others hear stories from friends or see misleading ads. Hispanic patients, in particular, are more likely to express concerns-42% in one FDA survey said they worried about quality. The key is to address these fears with clear visuals and simple language. The FDA’s infographic showing side-by-side manufacturing standards has helped many patients accept the switch.
Can I trust generics for chronic conditions like high blood pressure or diabetes?
Absolutely. Generics for chronic conditions like hypertension, diabetes, and high cholesterol are among the most widely prescribed and studied. A 2022 analysis of over 1 million patients showed no difference in outcomes between brand-name and generic statins, metformin, or lisinopril. The FDA’s approval process ensures that even for long-term use, generics perform identically. In fact, switching to generics often improves adherence because patients can afford to keep taking them.
What’s the difference between a generic and an authorized generic?
An authorized generic is made by the same company that produces the brand-name drug, often in the same factory, using the exact same ingredients and process. It’s sold under a generic label, usually at a lower price. A standard generic is made by a different company, but still meets FDA bioequivalence standards. Confusing the two is common-61% of physicians don’t know the difference. But both are safe and effective. The key is knowing which one you’re prescribing, especially when insurance switches patients.
Why aren’t generic prescribing tools built into my electronic health record?
Most EHR systems were built before generic education became a priority. Only 37% now include pop-up alerts for generic alternatives. But change is coming. The FDA launched a pilot in July 2023 connecting its generic drug database directly to Epic and Cerner. Early results show a 15.2% increase in generic prescribing among doctors using the integrated system. Ask your EHR vendor to add these alerts. If they say no, ask again in six months. Pressure from providers is what drives change.
How can I explain generic equivalence to a skeptical patient in under a minute?
Use this script: “This generic has the same active ingredient, same dose, and same effect as the brand. The FDA requires it to work the same way. The only difference is the price-it’s cheaper because it doesn’t need to pay for expensive marketing or repeat clinical trials. Many people have switched, and their results are exactly the same.” Then hand them the FDA’s one-page handout. It’s designed for exactly this moment.
Do generics have more side effects than brand-name drugs?
No. The FDA reviewed over 12,000 adverse event reports for generics in 2022 and found the rate was nearly identical to brand-name drugs. Side effects come from the active ingredient, not the pill’s color, shape, or brand name. Sometimes patients feel worse after switching-not because the drug changed, but because they expected to. That’s the nocebo effect. Clear communication reduces it.
Are there any drugs where generics aren’t recommended?
For most drugs, generics are recommended. But for complex formulations-like inhalers, topical creams, and certain extended-release pills-bioequivalence is harder to prove. In those cases, some doctors prefer to stick with the brand until more data is available. However, even for these, the FDA approves generics that meet strict standards. The issue isn’t safety-it’s complexity. Always check the FDA’s list of approved generics for your specific drug if you’re unsure.



Courtney Black
December 9, 2025 AT 19:13Generics aren't just cheaper-they're the quiet revolution in healthcare. We act like brand names have magic in the packaging, but the pill? Same molecule. Same effect. Same FDA stamp. The real drug isn't the brand-it's the science. And science doesn't care about logos.
iswarya bala
December 10, 2025 AT 04:56omg this is so true!! i live in india and generics saved my dad’s life-no joke, he was gonna stop meds cause cost, then we switched to generic metformin and he’s been fine for 3 years. why do we still act like brand = better? 🤦♀️
om guru
December 11, 2025 AT 09:31Prescribing generics is not merely a cost-saving measure. It is a clinical imperative grounded in pharmacological equivalence and public health ethics. The FDA’s bioequivalence standards are rigorous and non-negotiable. Failure to leverage generics constitutes a failure of evidence-based practice.
Tiffany Sowby
December 12, 2025 AT 02:23Wow. So now we’re supposed to trust pills made in some factory overseas with no brand name? Cool. Guess I’ll just keep paying $300 for my statin. At least I know who made it. The FDA doesn’t even inspect half the plants anymore. But hey, keep telling yourself it’s the same.
Asset Finance Komrade
December 12, 2025 AT 07:30Interesting. But let’s not romanticize generics. The 80–125% bioequivalence window is a legal loophole, not a scientific guarantee. Some patients *do* react differently-especially with narrow therapeutic index drugs. And yes, the system is broken. But blaming EHR vendors? That’s like blaming the pen for bad handwriting.
Brianna Black
December 13, 2025 AT 01:24Let me tell you about my cousin in Texas. She was on brand-name insulin for years. $1,200 a month. Then she switched to the generic. Same box. Same dose. Same results. She cried when she saw her first bill: $28. That’s not a drug change. That’s a dignity change. This isn’t about pills. It’s about people being treated like they matter.
Stacy Tolbert
December 14, 2025 AT 19:47I switched my mom to generic lisinopril last year. She was terrified. Said she’d ‘feel different.’ She didn’t. But she kept saying, ‘I just don’t trust it.’ That’s the real problem. Not the science. The fear. And we don’t talk about fear enough in medicine.
Ronald Ezamaru
December 15, 2025 AT 17:55The FDA’s data is solid, but the real win here is the toolkit. I printed the infographic and put it in my exam room. Patients stop asking me to justify it. They just read it. One elderly woman said, ‘So it’s like two different brands of aspirin?’ I said yes. She took it. No drama. No lecture. Just a picture. That’s all it took.
Ryan Brady
December 16, 2025 AT 05:5690% of prescriptions? Yeah, and 90% of those are made in China or India. You really think the quality control is the same? 🤨
Raja Herbal
December 17, 2025 AT 13:39Wow. So the answer to America’s healthcare crisis is… a PDF? And a pop-up? I guess we’ll just ignore the fact that insurance companies force switches before doctors even see the patient. But hey, here’s a cute diagram. Thanks for the laugh.