Most people think patent protection for drugs ends when the patent expires. But that’s not always true. In the U.S., the FDA can block generic versions of a drug for six extra months-even after the patent is gone-thanks to something called pediatric exclusivity. It’s not a patent extension. It’s not a new patent. It’s a regulatory pause, built into law, that gives drugmakers more time to sell their medicine without competition. And it’s one of the most powerful, yet least understood, tools in pharmaceutical lifecycle management.
How pediatric exclusivity actually works
Pediatric exclusivity comes from Section 505A of the Federal Food, Drug, and Cosmetic Act. It was created in 1997 and made permanent in 2002 under the Best Pharmaceuticals for Children Act. The goal? Get drug companies to study their medicines in kids. For decades, most drugs were only tested in adults. Doctors had to guess doses for children. Side effects were unpredictable. The law didn’t force companies to do pediatric studies. Instead, it gave them a powerful incentive: six months of extra market protection. Here’s the catch: the FDA doesn’t hand this out freely. A company must first get a Written Request from the FDA. That document spells out exactly what studies are needed-what age groups, what doses, what endpoints. The company then has to complete those studies, submit the data, and prove it was done exactly as requested. The FDA has 180 days to review it. If it passes, the six-month clock starts. But here’s the part most people miss: this six months doesn’t extend the patent. It extends the FDA’s ability to approve generic versions. Even if the patent expired yesterday, the FDA still can’t approve a generic drug for six more months. That’s because pediatric exclusivity blocks Abbreviated New Drug Applications (ANDAs) and 505(b)(2) applications-not the patent itself.It attaches to everything
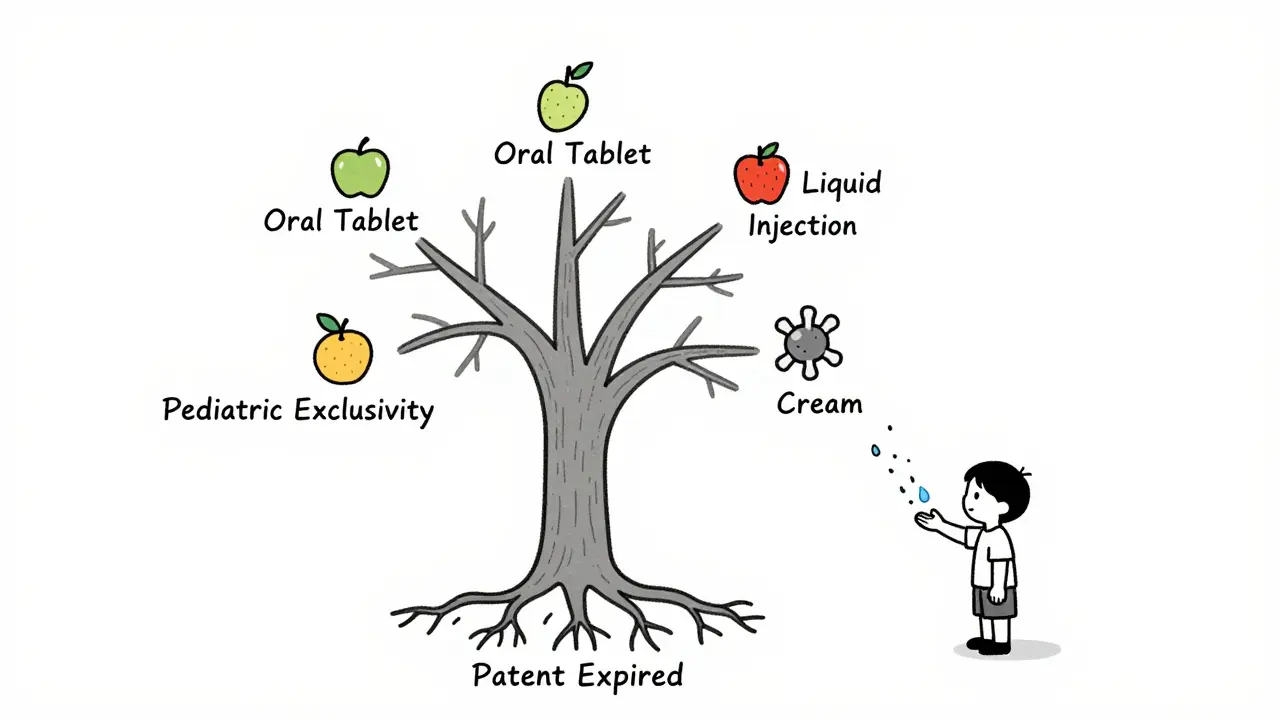
Pediatric exclusivity doesn’t just apply to the specific drug you studied. It applies to all dosage forms and indications of the same active ingredient. Let’s say a company makes an oral tablet for adults with high blood pressure. They get a Written Request to study it in children. They do the study. They get six months of exclusivity. Now, that six months also blocks generics for the injectable version, the liquid suspension, even the extended-release version-all if they contain the same active moiety. This is why it’s so valuable. A single pediatric study can lock out competition across an entire product line. The FDA’s own guidance says: “If a firm markets an oral formulation, a topical cream, and an ophthalmic solution containing the same active moiety, and all have remaining exclusivity or patent life, then 6 months additional exclusivity will be granted to ALL DOSAGE FORMS AND ALL INDICATIONS.”It extends other exclusivities too
Pediatric exclusivity doesn’t just ride on patents. It attaches to any existing marketing exclusivity the drug already has. That includes:- Five-year new chemical entity (NCE) exclusivity
- Three-year exclusivity for new clinical studies
- Orphan drug exclusivity (seven years for rare diseases)
It’s not a patent, but it blocks generics
This is where confusion sets in. Patents are legal rights enforced in court. Pediatric exclusivity is a regulatory tool enforced by the FDA. But the effect is the same: no generic can get approved. For generics, this creates a minefield. If they file an ANDA with a Paragraph IV certification (claiming the patent is invalid or not infringed), they can still be blocked by pediatric exclusivity-even if they win the patent lawsuit. The FDA’s position is clear: pediatric exclusivity stands on its own. A court ruling that a patent is invalid doesn’t automatically lift the exclusivity block. The generic company still needs a waiver from the brand company or a court order specifically lifting the exclusivity. And if the patent has expired? The FDA treats the generic application as a Paragraph II certification (patent expired). But pediatric exclusivity still blocks approval. That’s because the law says the FDA can’t approve any ANDA during the exclusivity period unless one of four conditions is met: (1) the brand company waives it, (2) a court rules the exclusivity doesn’t apply, (3) the brand company drops the lawsuit, or (4) the brand company wasn’t sued within 45 days of the ANDA filing.
Why biologics are left out
Pediatric exclusivity only applies to small-molecule drugs. It doesn’t apply to biologics-like insulin, monoclonal antibodies, or vaccines. That’s because biologics are regulated under a different law: the Biologics Price Competition and Innovation Act (BPCIA). Unlike the Hatch-Waxman Act, which governs small-molecule drugs, BPCIA doesn’t create patent linkage. That means FDA can approve biosimilars even if patents are still active, as long as they meet safety and efficacy standards. So, even if a biologic company does pediatric studies, they don’t get six months of extra market protection. That’s a major difference in how the two types of medicines are treated.Real-world value: millions at stake
For a blockbuster drug with $1 billion in annual sales, six months of exclusivity is worth $500 million. That’s why companies spend millions to get pediatric exclusivity. It’s not about helping kids-it’s about protecting revenue. And the FDA’s system is designed to make it hard to bypass. In one well-known case, a drug’s patent expired in 2018, but pediatric exclusivity blocked generics until 2019. The brand company didn’t have to change anything. No new patent. No new filing. Just a six-month delay that cost generics hundreds of millions in lost revenue. The FDA’s Orange Book reflects this clearly. Patents that are extended by pediatric exclusivity appear twice: once with the original expiration date, and again with the extended date. Generic manufacturers have to track both.What doesn’t trigger it
Pediatric exclusivity doesn’t come from:- Voluntary pediatric studies (unless requested by the FDA)
- Studies done outside the U.S.
- Studies that don’t follow the Written Request exactly
- Studies that only look at pharmacokinetics without clinical outcomes
How generics fight back
Generic companies know pediatric exclusivity is a hurdle. So they look for loopholes. One strategy is to challenge whether the exclusivity applies to their specific product. For example, if the brand company only studied the drug in children with a rare condition, but the generic wants to market it for a common condition, can the exclusivity still block them? The FDA says yes-if it’s the same active moiety. Another tactic is to file for a waiver. Some brand companies will grant waivers if the generic agrees to delay launch in other markets or pay a licensing fee. It’s a business deal, not a legal one. But the most effective way to beat pediatric exclusivity? Win a Paragraph IV lawsuit and get a court order that specifically says the exclusivity doesn’t apply. That’s rare. Courts usually defer to the FDA’s interpretation of the law.Is this fair?
Critics say pediatric exclusivity rewards companies for doing what they should have done anyway. Why should a drugmaker get six extra months of monopoly just because they studied their drug in kids? The answer: without this incentive, they wouldn’t do it. Before 1997, fewer than 20% of drugs had pediatric labeling. Today, it’s over 80%. That’s progress. The system isn’t perfect. Some companies do minimal studies just to get the six months. Others delay pediatric studies until just before patent expiry to maximize the benefit. But the result is clearer labeling, safer dosing, and better care for children.What’s next?
The FDA is now pushing for more real-world data in pediatric studies. They’re also exploring ways to streamline the Written Request process. But the core rule stays: no pediatric study, no six-month extension. And that six months? It’s still the most valuable regulatory tool in the drug industry-because it doesn’t require a patent. Just a well-done study.Does pediatric exclusivity extend the actual patent term?
No. Pediatric exclusivity does not extend the legal patent term. Instead, it delays the FDA’s ability to approve generic versions of the drug for six months, even after the patent expires. It’s a regulatory barrier, not a patent extension.
Can a generic drug be approved during pediatric exclusivity?
Only under four specific conditions: (1) the brand company grants a waiver, (2) a court rules the patent or exclusivity is invalid or not infringed, (3) the brand company drops its lawsuit, or (4) the brand company wasn’t sued within 45 days of the generic filing. Without one of these, the FDA cannot approve the generic.
Does pediatric exclusivity apply to biologics?
No. Pediatric exclusivity only applies to small-molecule drugs regulated under the Hatch-Waxman Act. Biologics, like insulin or monoclonal antibodies, are regulated under a different law (BPCIA) that doesn’t include pediatric exclusivity as a mechanism.
What happens if a drug has no patent or exclusivity left?
Pediatric exclusivity can still be granted if the pediatric study leads to a new approved indication. For example, if a drug was only approved for adults and the company studies it in children and gets a new pediatric label, that new application can qualify for six months of exclusivity-even if the original patent expired.
Does pediatric exclusivity apply to all forms of the same drug?
Yes. If a company studies one form of a drug (like an oral tablet) and earns pediatric exclusivity, it applies to all dosage forms and indications of the same active ingredient-whether it’s a cream, injection, or liquid. This makes it a powerful tool for protecting entire product lines.





Akshaya Gandra _ Student - EastCaryMS
January 3, 2026 AT 19:02wait so if i get this right the FDA just lets pharma companies lock down generics for 6 months just for doing kid studies? like... thats wild. i thought patents were the only thing blocking generics. this feels like a loophole big enough to drive a truck through.
Joseph Snow
January 4, 2026 AT 03:01Of course it’s a loophole. The entire pharmaceutical industry is a rigged casino. They don’t invent cures-they invent ways to extract money from sick people. Pediatric exclusivity? Just another tax on children’s healthcare. They study a drug in kids because they’re forced to-and then they milk it for half a billion dollars. This isn’t medicine. It’s corporate extortion.
Angie Rehe
January 4, 2026 AT 15:31Are you kidding me? They’re not ‘studying’-they’re gaming the system. The FDA gives them a checklist, they do the bare minimum to tick boxes, then sit back and collect six months of monopoly profits. And you call that ‘progress’? The only thing that improved is the bottom line of Big Pharma. Kids still get off-label prescriptions because no one actually tested dosing properly. This is performative science.
Jacob Milano
January 5, 2026 AT 23:57I get why people are angry, but let’s not throw the baby out with the bathwater. Before this law, kids were basically being treated as tiny adults-wrong doses, unknown side effects, zero data. Now? We have actual pediatric labeling on 80% of drugs. That’s huge. Yeah, the system’s flawed-but the outcome saved lives. Maybe we need better incentives, not to scrap the whole thing.
Ashley Viñas
January 6, 2026 AT 06:49Oh, so now we’re celebrating corporate compliance as ‘progress’? How quaint. The fact that you think this is a win says everything about your moral compass. If a company does what it’s *supposed* to do-study drugs in children-and gets rewarded with half a billion dollars? That’s not a policy. That’s a moral failure dressed in regulatory jargon.
Doreen Pachificus
January 6, 2026 AT 08:21So if a drug has no patent left, but they did the pediatric study, the FDA still blocks generics? That’s insane. I thought patents were the only legal barrier. This feels like a secret backdoor monopoly. Who even wrote this law?
Allen Ye
January 6, 2026 AT 16:06There’s a deeper philosophical question here: when does incentivizing good behavior become institutionalized corruption? We’ve created a system where the only reason a company does something ethically necessary-studying drugs in children-is because they’re paid in monopoly profits. That’s not a market. That’s a transactional morality. We’ve outsourced public health to corporate self-interest, and now we’re surprised when the system grinds children into profit margins. The real tragedy isn’t the six months-it’s that we’ve come to accept this as normal.
Cassie Tynan
January 6, 2026 AT 17:55Let me get this straight: the FDA lets a company lock down every single version of a drug-oral, injectable, cream, suspension-just because they studied one? That’s not exclusivity. That’s a corporate stranglehold. And they call it ‘science’? Please. This is legal cartels with lab coats.
Clint Moser
January 8, 2026 AT 03:10Wait… so if you’re a generic maker and you win a patent lawsuit, the FDA can STILL block you because of pediatric exclusivity? That’s not a loophole-that’s a constitutional violation. Who’s really running this country? The FDA? The DOJ? Or just the lobbyists who wrote this law in a back room at the Ritz-Carlton?
en Max
January 8, 2026 AT 23:39It is important to note, however, that pediatric exclusivity, as codified under Section 505A of the FDCA, operates as a distinct regulatory mechanism, entirely independent of patent law, and functions as a statutory bar to the approval of ANDAs and 505(b)(2) applications-regardless of patent status-provided the requisite criteria are met, including submission of data in strict compliance with a written request issued by the FDA’s Office of Pediatric Therapeutics. The system, while imperfect, has demonstrably increased the availability of labeled pediatric dosing information across therapeutic classes.
Enrique González
January 9, 2026 AT 18:38Look, I know it sounds shady, but if this is what it takes to get drugs studied in kids, then I’ll take it. I’ve seen kids get dosed wrong. I’ve seen parents panic because the label says ‘adults only.’ This system isn’t perfect-but it’s the only one that actually got pharma to stop pretending kids are just small adults. Let’s fix the abuse, not the solution.
Justin Lowans
January 10, 2026 AT 16:20Imagine if we applied this logic to everything: ‘Do your job, and we’ll give you a monopoly on your own product.’ That’s not innovation-that’s bribery. But hey, at least the kids got better labels. Maybe we should just pay them directly instead of letting CEOs buy yachts with the profits.
Michael Rudge
January 12, 2026 AT 08:18So… the FDA lets drug companies buy six months of monopoly power by doing… what? A single study? And they get to block every version of the drug? Even the ones they didn’t study? And biologics? Oh, they’re just… left out? Like they don’t matter? This isn’t science. This is a cartoon where the villain wins because he has the right lawyer.
Jason Stafford
January 13, 2026 AT 08:09They’re not studying kids-they’re studying how much money they can squeeze out of the system. This isn’t about children’s health. It’s about the FDA being owned by Big Pharma. The six-month delay? It’s not a reward. It’s a bribe. And the fact that you’re defending it? That’s the real scandal.