Generic drugs save patients and the healthcare system billions every year. In 2023, 90.7% of all prescriptions filled in the U.S. were for generics - yet they made up just 23% of total drug spending. That’s the power of competition. But behind those numbers is a quiet risk: not all generics perform the same. And pharmacists are often the last line of defense before a patient gets a medication that doesn’t work - or worse, causes harm.
What Makes a Generic Problematic?
A generic drug isn’t just a cheaper copy. It must contain the same active ingredient, strength, dosage form, and route of administration as the brand-name version. The FDA requires it to be bioequivalent - meaning it delivers the same amount of drug into the bloodstream within a specific range: 80% to 125% of the brand’s levels. That sounds tight. But for some drugs, even a 20% variation can be dangerous. Take levothyroxine, for example. It’s a thyroid hormone replacement. Too little, and a patient stays fatigued and hypothyroid. Too much, and they risk heart rhythm problems or bone loss. A 2021 study found that switching between different generic manufacturers of levothyroxine led to 2.3 times more cases of therapeutic failure than staying on one brand. One patient’s TSH level jumped from 2.1 to 8.7 after a switch - a change so dramatic, it required a full dose adjustment. The same goes for warfarin, phenytoin, and digoxin. These are called narrow therapeutic index (NTI) drugs. The window between effective and toxic is razor-thin. The FDA has flagged 18 such drugs as high-risk for substitution issues. Digoxin, in particular, has 12.7 adverse events per 10,000 prescriptions when switched between manufacturers - more than double the rate of non-NTI drugs.When Pharmacists Must Step In
Pharmacists don’t need to question every generic. Most work fine. But there are clear red flags. Here’s when to pause and investigate:- Patient reports sudden change in symptoms - especially within 2-4 weeks after a generic switch. If someone on levothyroxine suddenly feels exhausted again, or a patient on warfarin has unexplained bruising, don’t assume it’s just "bad luck."
- Therapeutic drug monitoring shows deviation - Blood levels of drugs like tacrolimus, cyclosporine, or phenytoin should stay stable. A drop or spike after a generic switch is a red flag. Document the manufacturer and report it.
- The generic is rated "BX" in the FDA’s Orange Book - This means the FDA has determined it’s not therapeutically equivalent to the brand. If you see a BX rating, don’t dispense it unless the prescriber specifically approves it. Most pharmacists don’t check this routinely - but they should.
- Complex formulations are involved - Extended-release tablets, inhalers, topical creams, and injectables are harder to copy. In 2020, FDA testing found 7.2% of generic extended-release opioids failed dissolution tests - meaning the drug didn’t release properly. That’s not a small number.
- Look-alike or sound-alike names - Oxycodone/acetaminophen vs. hydrocodone/acetaminophen. These mix-ups cause 14.3% of all generic medication errors, according to the Institute for Safe Medication Practices. Always double-check the label, even if the prescription looks familiar.
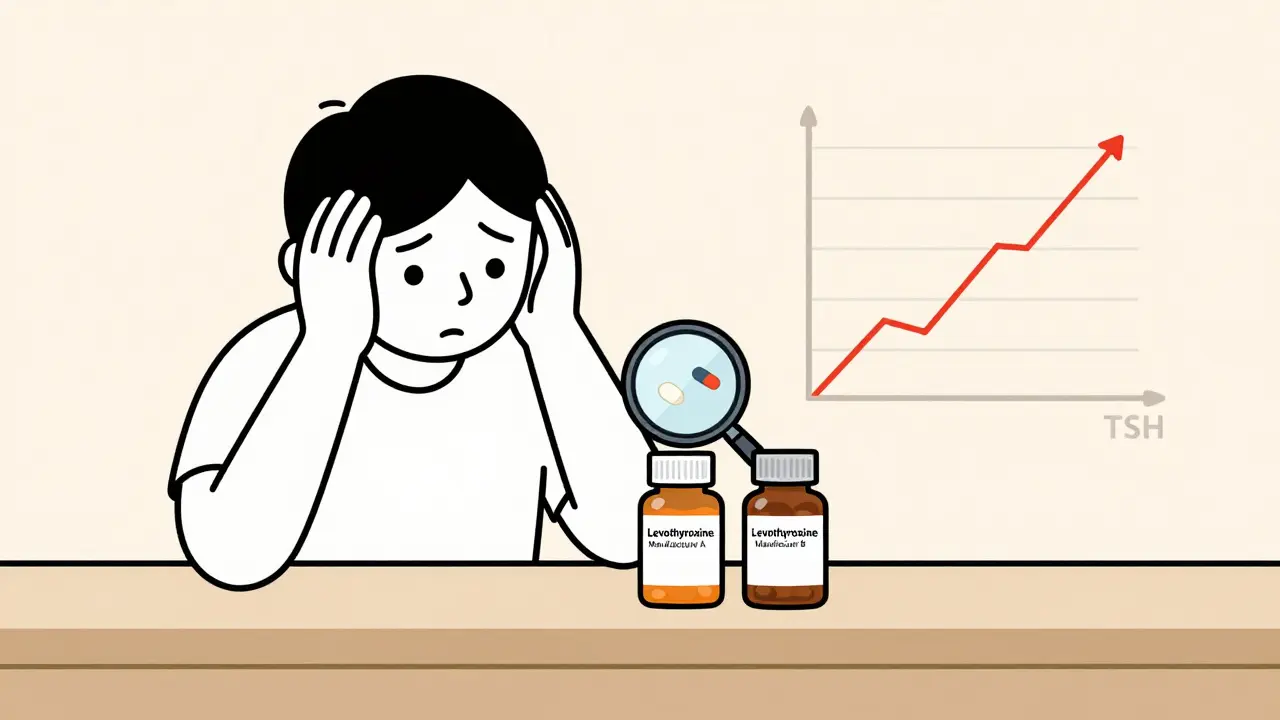
The Hidden Risks of Switching Manufacturers
Patients often don’t realize they’re getting a different generic. Pharmacies switch suppliers for cost reasons - and patients rarely ask. But switching manufacturers, even within generics, can cause real problems. A 2023 Consumer Reports survey found that 22.4% of patients reported different side effects after switching between generic brands. One patient described feeling dizzy and nauseous after a switch from one generic metformin to another. Another said their acid reflux got worse after changing generic omeprazole brands - even though the label said "same active ingredient." Why? Because inactive ingredients - fillers, dyes, coatings - can affect how the drug dissolves. For delayed-release drugs like proton pump inhibitors, a different coating can mean the pill breaks down too early or too late. That’s why 32.8% of patient complaints about generics involve gastrointestinal meds. And it’s not just patient reports. The FDA’s MedWatch database received 1,842 patient submissions between 2020 and 2023 about inconsistent effectiveness or unexpected side effects from generics. Nearly 40% of those came from people who switched manufacturers.What Pharmacists Can Do - And Must Do
You don’t need to be a chemist to spot trouble. Here’s what works:- Track the manufacturer - Write the manufacturer name on the prescription label or in the patient’s record. If a problem arises, you’ll know which batch to flag. Studies show 68.4% of therapeutic failure investigations require manufacturer-specific data.
- Use the FDA’s Orange Book - Look up the drug. Is it rated "AB"? That’s good. Is it "BX"? Don’t dispense unless the prescriber overrides it. Many pharmacies don’t check this - but you should.
- Ask patients - Don’t assume they know what they’re taking. Ask: "Have you noticed any changes since your last refill?" or "Did you feel different after your last prescription?"
- Report it - Use the FDA’s MedWatcher app. It takes less than five minutes. Every report helps the FDA find patterns. Between 2020 and 2022, pharmacist-reported incidents rose 18.3% in states with mandatory reporting.
- Know your state’s laws - In 29 states, pharmacists must substitute generics unless the prescriber says "do not substitute." But in four states - Massachusetts, New York, Texas, and Virginia - there are special rules for NTI drugs. You can’t switch them without explicit permission.

Why This Matters - Beyond Cost
Cost savings are real. But patient trust is fragile. When a generic doesn’t work, patients lose faith - not just in the drug, but in the system. They start refusing generics altogether. They pay more. They skip doses. They go to the ER. The FDA is responding. In 2023, they launched the Complex Generic Products Team and issued 28 new guidances for hard-to-copy drugs. They’re also planning to increase generic drug testing by 40% over the next three years. But that’s long-term. What happens tomorrow? That’s where you come in. You’re not just filling prescriptions. You’re monitoring safety. You’re the one who notices when a patient’s blood pressure suddenly spikes after switching from one generic lisinopril to another. You’re the one who remembers that the last batch of generic metoprolol made three patients feel faint. You’re the one who checks the Orange Book before dispensing a BX-rated generic. It’s not about distrust. It’s about vigilance.What’s Next?
The FDA plans to use artificial intelligence to scan adverse event reports and flag problematic generics before they cause widespread harm. That’s promising. But AI can’t replace human judgment. It can’t ask a patient how they’re feeling. It can’t notice that the pill looks different - even if the label says it’s the same. So keep doing what you do. Track. Ask. Report. Educate. Don’t wait for a crisis. If a patient says, "This doesn’t feel right," listen. It might be the only warning the system gets.Are all generic drugs safe?
Most generics are safe and effective - about 90% work just like their brand-name counterparts. But a small percentage can cause problems, especially for drugs with a narrow therapeutic index like levothyroxine, warfarin, or digoxin. Bioequivalence standards allow for up to 20% variation in drug absorption, which can be enough to cause therapeutic failure in sensitive patients.
How do I know if a generic is rated "BX"?
Check the FDA’s Orange Book, which lists all approved drugs and their therapeutic equivalence ratings. "AB" means therapeutically equivalent. "BX" means not equivalent - often due to unresolved bioequivalence concerns or complex formulations. If you see a BX rating, do not substitute unless the prescriber explicitly approves it. Many pharmacy systems don’t flag BX ratings automatically, so you need to check manually.
Can I switch a patient back to the brand-name drug?
Yes - but only if the prescriber agrees. If a patient has a documented adverse reaction or therapeutic failure after a generic switch, document it thoroughly and contact the prescriber. In some cases, especially with NTI drugs, the prescriber may write "dispense as written" or "do not substitute." Pharmacists are not allowed to override that without authorization.
Why do some generics cause different side effects?
While the active ingredient is the same, inactive ingredients - like fillers, dyes, and coatings - can vary between manufacturers. These can affect how the drug dissolves or is absorbed. For example, a delayed-release omeprazole pill from one maker might break down too early in the stomach, causing heartburn instead of relieving it. These differences are small, but they matter to sensitive patients.
Should I always tell patients which manufacturer made their generic?
It’s not required, but it’s a best practice. Many patients don’t realize they’ve been switched. If you note the manufacturer on the label or in the patient’s record, it helps if they report side effects later. It also makes it easier to trace problems back to a specific batch if multiple patients report the same issue.






Erika Putri Aldana
December 21, 2025 AT 02:15This is why I never take generics anymore. 😒 My last one made me feel like a zombie for two weeks. They're all the same on paper, but your body knows the difference. Pharma doesn't care as long as they're making money.
Jon Paramore
December 22, 2025 AT 23:55NTI drugs are the real issue here. The 80-125% bioequivalence window is mathematically sound for most compounds, but for drugs like warfarin or levothyroxine, that range translates to clinically significant fluctuations in plasma concentration. The FDA’s AB/BX rating system exists for a reason - pharmacists need to be trained to interpret it, not just rely on automated substitution protocols.
Hannah Taylor
December 24, 2025 AT 00:27you ever wonder why the same generic from the same company tastes different sometimes? they're adding fluoride or something. i think the gov't is testing mind control drugs in our prescriptions. i saw a documentary. also, my neighbor's dog got sick after taking his meds. coincidence? i think not.
Swapneel Mehta
December 24, 2025 AT 05:38Really appreciate this breakdown. As someone who’s seen patients struggle with switching generics, it’s frustrating that cost drives decisions more than outcomes. But small actions - like noting the manufacturer on the label - can make a huge difference. Keep speaking up, pharmacists. You’re the unsung heroes.
Dan Adkins
December 25, 2025 AT 21:15It is imperative to underscore that the regulatory framework governing generic pharmaceuticals is fundamentally inadequate for ensuring therapeutic consistency across all drug classes. The current bioequivalence paradigm, predicated on mean AUC and Cmax tolerances, fails to account for inter-individual pharmacokinetic variability, particularly in elderly and polypharmacy populations. A paradigm shift toward individualized bioequivalence thresholds is not merely advisable - it is ethically obligatory.
Teya Derksen Friesen
December 26, 2025 AT 18:53Every pharmacist should be required to complete a mandatory module on NTI drugs before they can dispense. This isn’t optional safety - it’s core to our professional duty. We’re not just technicians. We’re patient advocates. And if we don’t push back when something’s off, who will?
Sandy Crux
December 28, 2025 AT 17:19...And yet, somehow, we're still expected to trust the FDA's "Orange Book"... which, by the way, hasn't been meaningfully updated since 2019... and, let's be honest, it's mostly just a glorified spreadsheet that Big Pharma helped write... and don't even get me started on the fact that "AB" ratings are sometimes given to generics that have been recalled in Europe... but hey, let's just keep pretending everything's fine... 🙃
Jay lawch
December 28, 2025 AT 19:49Let me tell you something. In India, we make 40% of the world’s generics. And you think they’re cutting corners? No. We’re making them better. You Americans think your FDA is perfect? Look at the opioid crisis. Look at the Vioxx scandal. Look at the fact that your brand-name drugs cost ten times more than ours - and they’re the same damn pills! Your system is broken. You blame generics because you don’t want to face the truth: your healthcare system is a scam. We make safe, effective drugs. You just can’t afford to admit it.
Christina Weber
December 29, 2025 AT 02:23It is a gross dereliction of professional duty for pharmacists to fail to document manufacturer-specific adverse events in patient records. The absence of such documentation directly impedes pharmacovigilance and constitutes a breach of the standard of care. Furthermore, failure to consult the FDA Orange Book prior to dispensing constitutes negligence under the Model Pharmacy Act § 12.7(c). This is not opinion - it is legal and ethical obligation.
Orlando Marquez Jr
December 30, 2025 AT 17:38In many African and Southeast Asian nations, pharmacists routinely maintain handwritten logs of generic manufacturer and lot numbers - not because they’re required, but because they’ve learned from experience. We have much to learn from their grassroots vigilance. Technology can help, but human attention remains irreplaceable. Thank you for highlighting this critical, underappreciated role.